Due to the possible formation of a set of regioisomers in chemical reactions, controlling the regioselectivity has been a fundamental issue in organic synthesis.
Organosilanes are widely used in silicon rubbers, paper releasing coatings, and pressure-sensitive adhesives. Catalytic hydrosilylation of alkenes represents a simple strategy for the synthesis of organosilanes.
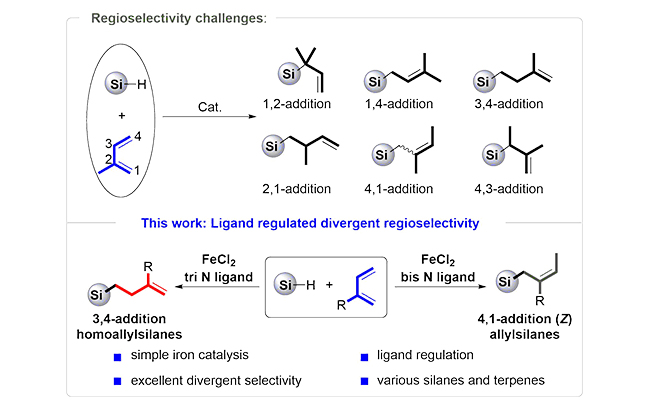
So far, the catalytic hydrosilylation of alkenes focused more on simple alkenes while less on hydrosilylation of naturally abundant terpenes because it's hard to differentiate four electronically unbiased alkenylcarbons on isoprene.
Regiodivergent hydrosilylation of isoprene (Image by KUAI Chang-Sheng)
Recently, a research team led by Prof. CHEN Qing'an from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) proposed a novel "ligand regulation" strategy to realize iron-catalyzed regiodivergent isoprene hydrosilylation.
Their findings were published in Angew. Chem. Int. Ed.
The key to success relies on the coordination number of the ligand to the iron center. The utilization of tri-N ligand enabled the formation of 3,4-adducts homoallylsilanes, whereas varying to bis-N ligand switched the selectivity to 4,1-adducts allysilanes.
This study contributes to the art of regioselectivity control in alkene hydrofunctionalization. It was supported by National Natural Science Foundation of China. (Text by KUAI Chang-Sheng)