The geometric phase (GP) effect is an important quantum dynamical phenomenon. It represents the phase acquired when a quantum state is adiabatically transported around a closed circuit in the parameter space while remaining in the same Hilbert space.
In a molecule, the presence of conical intersection leads to the appearance of the GP effect in the adiabatic ground state. In the previous studies, the influence of the GP effect on molecular spectrum has been well understood. However, how the GP acts on the chemical reaction remains unclear.
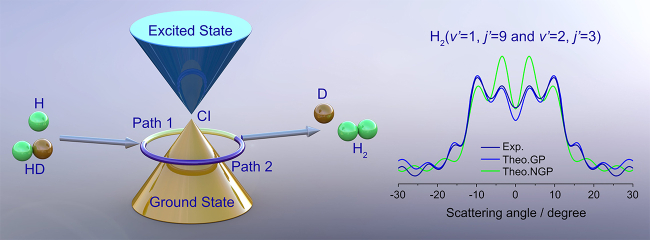
Observation of the geometric phase effect in the H+HD→H2+D reaction below the conical intersection (Image by HUANG Yin)
Recently, Prof. SUN Zhigang and Prof. YANG Xueming from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. WANG Xingan from University of Science and Technology of China, observed the GP effect in the H+HD→ H2+D reaction below the conical intersection (CI).
This study was published in Nature Communications on July 20.
"We focused on the case of the reaction energy lower than the CI. In this case, the GP effect became much weaker and was more difficult to observe." said Prof. SUN.
The scientists observed the GP effect in H+HD→ H2+D reaction at 2.28 eV, which was well below the CI, by further improving the high-resolution time-sliced velocity map imaging-CMB technique and developing quantum dynamical method based on hyperspherical coordinates. The GP effect was clearly identified by the observation of distinct oscillations in the differential cross section around the forward direction.
Quasi-classical trajectory analysis revealed that GP effect was caused by the interference between usual direct abstraction reaction pathway and roaming-like abstraction reaction pathway.
This study proved soundly the existence of the GP effect in a chemical reaction after adaptation of the Born-Oppenheimer approximation, and the influence of GP on chemical reaction was manifested by oscillations of differential cross section.
The work was highly praised by reviewers. One reviewer commented: "I regard this study as an outstanding advance in this classic (but not classical!) reaction system. It is the stuff that should go into future textbooks. As someone who tried but failed to observe the same phenomenon, I am in awe of what has been accomplished." The second reviewer also commented: "This experiment has been the dream of at least one generation of physical chemists and these results are simply beautiful… I believe this work will be included in physical chemistry textbooks, since it demonstrates a fundamental quantum feature on chemical reaction dynamics."
The study was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Strategic Priority Research Program of Chinese Academy of Sciences. (Text by HUANG Yin)