Recently, Prof. YIN Heng and Prof. LI Guohui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences discovered a novel interaction pattern of lytic polysaccharide monooxygenase (LPMO) with cellulose and determined the key residues involving in the process.
Their study, pulbished in J. Phys. Chem. Lett., sheds light on mechanisms of substrate preferences and regioselectivities of the enzyme.
Enzyme-substrate interaction is essential to elucidate the catalytic mechanism of enzymes and guide enzyme engineering. However, the interaction of the enzymes with their insoluble substrates in the two-phase interface has always been a tremendous problem due to the inapplicability of traditional methods.
The study of polysaccharide biodegradation catalyzed by LPMOs is one of these instances. LPMOs are a class of copper dependent metalloenzymes that oxidatively break down recalcitrant polymeric carbohydrates and provide more sites for glucoside hydrolase acting on.
Prof. YIN has been committed to mining novel LPMOs, identifying activity, and constructing light-driven LPMO activation. However, the mechanisms of the interactions between these enzymes and their substrates have yet to be fully elucidated.
In this study, the scientists employed computational and biochemical approaches and discovered that the enzymes could stably bind on the flat hydrophobic surface of cellulose via the interactions of the several key residues with two adjacent cellulose chains.
Combining with previous studies, they generated a hypothesis that the position and orientation of the bound enzymes on the surface of cellulose might be related to their regioselectivity.
Further study on the correlation of on the correlation of substrate binding and H2O2 accumulation suggested that the oxidation of the insoluble polysaccharides catalyzed by LPMOs was mainly driven by H2O2.
"These findings broadened our knowledge of the interaction between enzymes and insoluble substrates and deepened our understanding of the role that H2O2 plays in LPMO activity." said Prof. YIN.
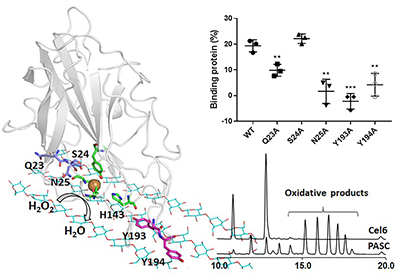
The interactions of LPMO with cellulose and the key residues. (Image by ZHOU Haichuan)
This work was supported by the Chinese National Nature Science Foundation, CAS Youth Innovation Promotion Program, Liaoning Revitalization Talents Program and DICP Innovation Fund. (Text by ZHOU Haichuan)