Research group led by Prof. XU Guowang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, in collaboration with the research group from The First Affiliated Hospital of Zhengzhou University and National Institutes of Health, U.S.A. achieved a new progress in the functional metabolomics and translational medicine study of Acute-On-Chronic liver failure treatment. This work was published in the journal Advanced Science.
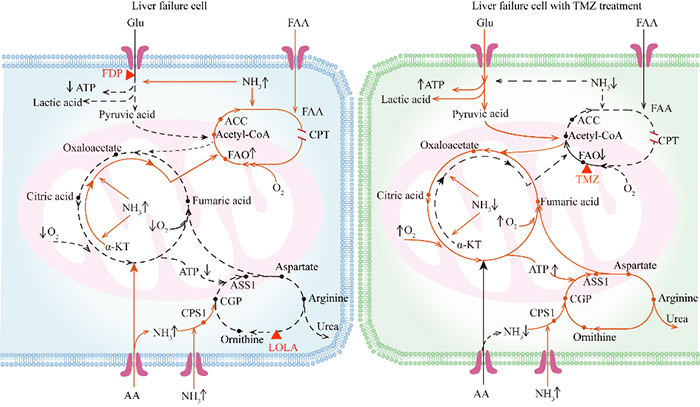
The schematic of the metabolic reprogramming of liver failure cell (left) and liver cell with TMZ treatment (right). (Image by ZHOU Yang)
Acute-on-chronic liver failure (ACLF) is characterized by acute decompensation of liver function, organ failure(s), and high mortality. The main cause of ACLF in Asia is chronic HBV infection.
Liver transplantation remains the only effective therapy, but a high mortality rate of patients on the waiting list is mainly caused by the rapid disease progression and the lack of donors. Thus, it is urgent to find an effective therapeutic strategy for patients with ACLF.
In view of this problem, the metabolic reprogramming and intervention of liver failure were studied by using the strategy of functional metabolomics. By analyzing metabolic profiles in liver tissue samples from HBV-related ACLF patients and the controls, the metabolic characteristics of HBV-related ACLF patients were identified: inhibited glycolysis, tricarboxylic acid cycle and urea cycle, and enhanced fatty acid oxidation (FAO) and glutamine anaplerosis.
These effects were mainly attributed to hyperammonemia and hypoxia. Further in vitro study revealed that switching from FAO to glycolysis could improve hepatocyte survival in the hyperammonemic and hypoxic microenvironment. Based on our insight into the mechanism of metabolic reprogramming in patients with ACLF, a novel treatment strategy was proposed for patients with ACLF: inhibiting FAO.
Trimetazidine (TMZ) is a specific inhibitor of FAO that attenuates cardiomyocyte injury by switching the energy metabolism from FAO to glycolysis and has been used for anti-angina therapy.
As expected, this study found that TMZ increased glycolysis, as evidenced by increased pyruvate levels and high extracellular acidification rate (ECAR) at maximal capacity, in the Chang liver cells exposed to hyperammonemia, which was accompanied by reduced ammonia levels.
Intriguingly, it also increased the oxygen consumption rate (OCR) at both the basal level and maximal capacity and increased ATP generation, which suggested an enhanced oxidative phosphorylation Furthermore, TMZ effectively decreased the apoptosis rate in liver tissue from rats exposed to NH4Cl. A randomized clinical trial (ChiCTR-OPC-15006839) confirms that inhibiting FAO using TMZ improves the prognosis of patients with HBV-related ACLF, especially severe ACLF patients.
In conclusion, this study provides a practical strategy for targeting metabolic reprogramming using TMZ to improve the survival of patients with HBV-related ACLF.
This work was supported by the National Natural Science Foundation of China, National Key Research and Development Program of China. (Text by ZHOU Yang)