A joint research team led by Prof. HUANG Jiahui Huang and Prof. QIAO Botao Qiao from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), and Prof. SUN Keju Sun from Yanshan University made an important progress in gold nanocatalysis.: They developed an anti-sintering gold nanocatalyst with high catalytic activity. The results were published in Nature Communications.
Gold nanocatalysts have exhibited unexpected catalytic activities in many catalytic reactions such as CO oxidation, propylene epoxidation and selective oxidation of alcohols/aldehydes, and thus been be regarded as one kind of promising catalysts for industrial application.
However, the commercialization process of gold nanocatalysts is very slow. A major barrier is their low stability stemmed from the easy sintering of gold nanoparticles. Therefore, the development of gold nanocatalysts with excellent stability becomes one of the biggest challenges in nanogold catalysis field.
Many efforts have been focused on addressing the sintering issue of gold nanocatalysts and signi?cant progresses have been achieved. Strategies such as using the strong interaction between the metal and support, coating the catalysts by inert oxide, utilizing meso-porous materials to con?ne noble metal particles can effectively improve the sintering resistance of gold nanocatalysts. However, these progresses were achieved at the cost of losing the activity to different extent.
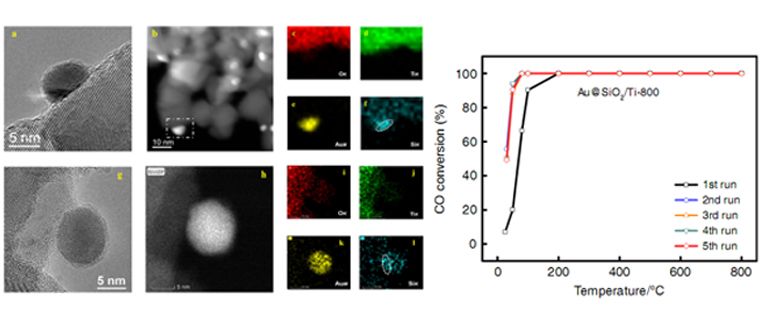
STEM images and EDS mapping of SiO2 modified gold nanocatalyst (Left) and CO conversion versus temperature for different cycles (Right). (Image by ZHANG Junying)
Recently, the joint research team prepared a SiO2 modified gold nanocatalyst , by co-deposition of gold and silica precursors on the TiO2 support and subsequent high temperature calcination. This method realizes the mixing of gold species and silica species in atomic level. Through the subsequent calcination process, a SiO2 film with only a thickness of a few atom layers was formed, which was found to cover the surface of gold nanoparticles.
This catalyst exhibited highly sintering resistant property and gold nanoparticles could maintain at about 6 nm even after 800 °C calcination. This catalyst also displayed excellent catalytic property and could realize 100% conversion of CO at 0 °C in CO oxidation.
Experiments together with computational studies revealed that the SiO2 layer over gold nanoparticles not only prevented the growth of gold nanoparticles, but also promoted the adsorption and activation of O2 during CO oxidation, resulting in a high catalytic activity. The ?nding paves a way for the design and development of gold nanocatalysts with excellent stability and high catalytic activity.
This work is supported by National Natural Science Foundation of China and “Transformational Technologies for Clean Energy and Demonstration”, Strategic Priority Research Program of the Chinese Academy of Sciences. (Text and Image by ZHANG Junying)