Research group led by prof. XU Zhaochao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, as well as LIU Xiaogang’s group from Singapore University of Technology and Design have recently reported a reliable prediction method for twisted intramolecular charge transfer (TICT) formations in various fluorophores. Their results were published on Angew. Chem. Int. Ed.
TICT is a general photophysical process that can significantly quench the fluorescence and reduce the photo-stability of a dye. During such process, the donor or acceptor fragment will twist towards a perpendicular molecular conformation, resulting in a non-emissive and completely charge-separated species.
Therefore, the suppression of TICT can greatly enhance the fluorescence intensity and photo-stability, meeting the requirements of modern single molecular biosensing and super resolution bioimaging. However, it is still challenging to accurately predict the TICT formations in various systems towards the quantitative design of bright and stable fluorophores.
Researchers aim to deeply understand and explore the unique fluorescence mechanisms by combining experiments and calculations. Based on the preliminary mechanistic understanding (J.Am. Chem. Soc., 2016, 138, 6960-6963; Angew. Chem. Int. Ed., 2019, 58, 7073-7077), they realized accurate predictions in various TICT-related fluorophores.
According to the nature of TICT formation, researchers summarized S1 potential energy surfaces (PESs) of 13 types of fluorophores. They found the rotation barrier (ERB) and driving energy (EDE) is the key in describing TICT formations.
In particular, the fluorophore is more likely to stay in its bright state (LE or ICT states) with the larger ERB and EDE; If ERB > 0 and EDE < 0, the fluorophore will partly form TICT states; If ERB = 0 and EDE < 0, the fluorophore will significantly form TICT states, resulting in the substantial quenching of fluorescence. In short, the TICT formations can be accurately predicted via ERB and EDE.
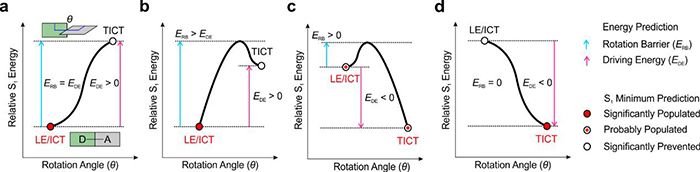
Different types of S1 potential energy surface. (Image by WANG Chao)
Then the researchers designed PRODAN derivatives and AIE functional materials to validate the TICT predictions. From the plot of S1 PES of PRODAN, they found that N-TICT will occur in water, while O-TICT will be prohibited. The comparison of S1 PES in different fluorophores suggest that the introduction of azetidine functional group in P4 can suppress TICT due to large ERB (0.38 eV) and small magnitude of EDE (-0.14 eV).
The experiment indicates that the quantum yield of P4 (Φ = 0.38) is about twice of P2 (introduction of dimethylamino). The authors also validated the existence of TICT in both P2 and P4 with different degree of fluorescence quenching. In comparison to dimethylamino, the introduction of azetidine effectively suppresses the TICT states. They validated the reliability of prediction method and concluded the 20-years mechanistic debates in PRODAN: PRODAN forms TICT states in water resulting in the quenching of fluorescence (other quenching mechanism may also exist).
This work is supported by National Natural Science Foundation of China and Chinese Academy of Sciences Special Research Assistant Project. (Text by WANG Chao ).