Recently, the research group led by Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Scientists (CAS) has made a new progress in highly selective electroreduction of CO to ethylene. This work was published as a communication in Angew. Chem. Int. Ed. and was highlighted as the Hot Paper.
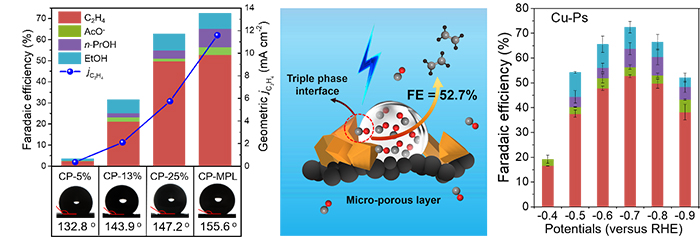
Performance of CO electroreduction over Cu-Ps loaded on hydrophobic carbon papers and schematic illustration for CORTE process on Cu catalysts with the assistant of the hydrophobic micro-porous layer to improve the CO diffusion. (Image by CHEN Ruixue, GAO Hehua)
Ethylene (C2H4) is an important building block for chemical industry and usually be produced by steam cracking of naphtha feedstocks at 800-900 oC worldwide. However, considering the utilization of the nonpetroleum carbon resources, converting CO from syngas (CO + H2) to C2H4 is also regard as one of the key process.
Fischer-Tropsch synthesis (FTS) is usually under high temperature (200-450 oC) and high pressure (5-50 bar), the products from FTS process are often limited by the Anderson-Schulz-Flory distribution, which leads to 30% selectivity of C2 hydrocarbons at most.
Therefore, the development of highly selective and energy-saving ethylene production route without additional separation and waste of carbon resources is of great technological, economical and environmental interest.
A highly selective production of ethylene process from electrocatalytic CO reduction with water at room temperature and ambient pressure was achieved through rational optimization of polytetrafluoroethylene content to increase CO concentration at the surface of electrode and Cu particle catalysts to enhance the C-C bond coupling.
This electrocatalytic CORTE process can achieve an unprecedentedly high ethylene FE of 52.7%. Moreover, the selectivity based on CO conversion to ethylene is around 70%, breaking through the 30% selectivity limitation from CO to C2 hydrocarbons in traditional FTS process, which always delivers uncontrollable mixture of C1-C4 hydrocarbons and massive CO2.
Besides, cooperating with Prof. SU Haiyan and Prof. ZHANG Donghui, DENG’s group studied the reaction active sites and mechanism by DFT calculations. Theoretical calculations suggest that Cu(100) is the most preferred plane for C2H4 formation via considering all possible products.
This electrocatalytic CORTE process provides a highly selective, energy-efficient and eco-friendly way to convert the abundantly industrial CO to C2H4 in comparison with the Fischer-Tropsch synthesis process.
This work was supported by Ministry of Science and Technology of China, National Natural Science Foundation of China, Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, the DNL Cooperation Fund and National Postdoctoral Program for Innovative Talents, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM). (Text by CHEN Ruixue and GAO Hehua)