Recently, a research group led by Prof. DONG Wenrui and Prof. YANG Xueming from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) directly measured the unimolecular reaction rate of syn-CH3CHOO. The results show the decomposition of syn-CH3CHOO could be one of a major source of atmospheric OH radicals. This work was published in The Journal of Physical Chemistry Letters.
OH radicals can oxidize most of the atmospheric species, therefore it is a crucial chemical cleaning agent of the atmosphere. Day-time OH radical production is dominated by the photolysis of ozone and subsequent reactions.
Until recently, ozonolysis of alkenes was thought to be another important source of atmospheric OH, through the decomposition of Criegee intermediate (mainly the syn-conformation) from the ozonolysis reaction.
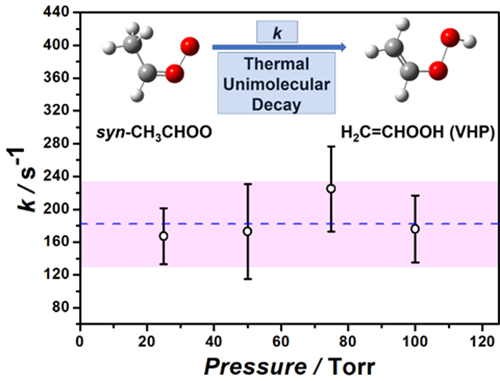
Unimolecular reaction rates of syn-CH3CHOO at various pressures. (Image by ZHOU Xiaohu)
Although syn-CH3CHOO is the simplest syn-conformation Criegee intermediate, its unimolecular reaction rate has not been accurately measured due to the interference from anti-CH3CHOO. Thus, the formation rate of OH from the decomposition of syn-CH3CHOO remains uncertain.
In this work, scientists developed a high-repetition-rate laser-induced fluorescence method to exclusively study the kinetics of syn-CH3CHOO. The results show that syn-CH3CHOO is mostly consumed by its unimolecular reaction and produces a substantial amount of OH radicals under atmospheric condition.
This method may have broad applications in measuring the kinetics of CIs, especially in that of some conformers of the larger CIs where other well-established methods encounter limitations.
The work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, the Chemical Dynamics Research Center, and the National Natural Science Foundation of China. (Text by ZHOU Xiaohu)