A research group led by Prof. LIU Jian from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) presented a new methodology for the preparation of monometallic/metal oxide confined within nitrogen-doped hollow carbon capsules. They applied this non-precious metal catalytic system to the hydrogenation of nitrobenzene to aniline. The study was published in Advanced Science.
The catalytic hydrogenation nitroarenes into their substituted anilines are importance for the chemical industry such as the production of dyes, pharmaceuticals, pigments, and agrochemicals. However, catalysts currently used in industry have low reactivity and poor hydrogenation selectivity.
Although noble-metal catalysts have been proved to be very efficient for catalytic hydrogenation reactions, they are expensive and rare. Therefore, it is necessary to develop transition metals that are cheap, abundant and can improve catalytic activity.
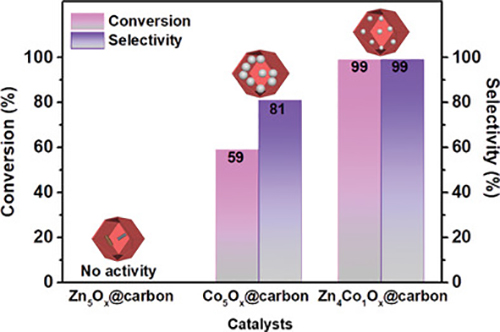
Catalytic performance of catalysts with different Zn/Co ratio. (Image by TIAN Hao)
The scientists obtained yolk-shell structured Co-CoOx@N-C by coating polymers on the surface of a series of Zn/Co bimetallic zeolite imidazole framework (Zn/Co ZIF), followed by hydrothermal treatment and confined pyrolysis.
Compared with monometallic Co ZIF, the introduction of inactive Zn nanoparticles could suppress the sintering of the Co species and enhance their dispersion in the carbon structures. Moreover, the strong interaction between the cobalt nanoparticles and the surrounding carbon shell were greatly enhanced by the presence of Zn within the Co structure, which provided positive synergistic effects and served to better disperse the Co particles.
The specific surface area and Co particle size were optimized through finely tuning the original Zn content in ZIF particles, thus enhancing overall catalytic activity. The yolk-shell structured Zn4Co1Ox@carbon hollow capsules were shown to be a highly active and selective catalyst (selectivity >99%) for hydrogenation of nitrobenzene to aniline. Furthermore, Zn4Co1Ox@carbon hollow particles showed superior catalytic stability, and no deactivation after 8 cycles of reaction.
The hollow Co-CoOx@N-C capsules might provide a pathway for green and sustainable catalytic process for fine chemicals production.
This work was supported by Dalian National Laboratory For Clean Energy Cooperation Fund, Chinese Academy of Sciences, Liaoning Talent Program. This article was dedicated to the 70th anniversary of DICP. (Text by WANG Xinyao and TIAN hao)