Solar energy, with tremendous potential as an environmentally sound and sustainable energy source, dwarfs all the derivative sources by a wide margin. The challenge, however, is to take full advantage of the abundant and infinite solar energy and to convert it into readily utilisable and storable forms.
Solar thermochemical CO2 and H2O splitting (STCDS, STWS), tapping sunlight directly and storing solar energy in renewable fuel, are emerging technologies towards meeting this goal. Successful adoption of solar-to-fuel technologies is predicated upon identifying advanced materials with higher efficiency.
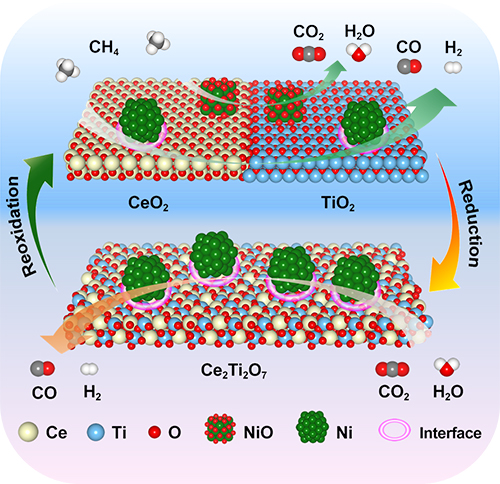
Schematic of the proposed reaction mechanism for MDR-STCDS and MDR-STWS processes over 5Ni/CeO2-TiO2 redox catalyst. (Image by RUAN Chongyan)
Recently, a research group led by Prof. WANG Xiaodong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences developed a Ni promoted ceria-titanium oxide catalytic system for highly effective thermochemical CO2 and H2O splitting as well as partial oxidation of CH4 at 900 °C.
The CO and H2 production rates and productivities were 10-140 and 5-50 times higher than the current state-of-the-art STCDS and STWS processes, respectively. Close to complete CH4 conversions with high selectivity towards syngas were achieved in the reduction step. Their findings were published online in Energy Environ. Sci..
By combining detailed experimental characterization with density functional theory (DFT) calculations, Prof. WANG Xiaodong and his collaborators from Xi’an Jiaotong University shed light upon the underlying mechanism for the exceptional reaction performance.
It was revealed that the metallic Ni and the Ni/oxide interface manifested the catalytic activity for both CH4 activation and CO2 or H2O dissociation, whereas CeO2-TiO2 enhanced the lattice oxygen transport via the CeO2-TiO2 / Ce2Ti2O7 stoichiometric redox cycle for CH4 partial oxidation and the subsequent CO2 or H2O splitting promoted by catalytic active Ni.
“We anticipate the fundamental understanding on the crucial roles of the catalytic sites for reactants activation and the stoichiometric redox chemistry for enhanced lattice oxygen availability can provide important guidance for the rational design of advanced materials toward solar fuel production,” said Prof. WANG. (Text by RUAN Chongyan)