The research teams led by Prof. ZHAO Zongbao K. and Prof. XUE Song from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences revealed the molecular bases of phosphite dehydrogenase (Pdh) variants binding to a non-natural cofactor nicotinamide cytosine dinucleotide (NCD). Their findings were published in ACS Catalysis.
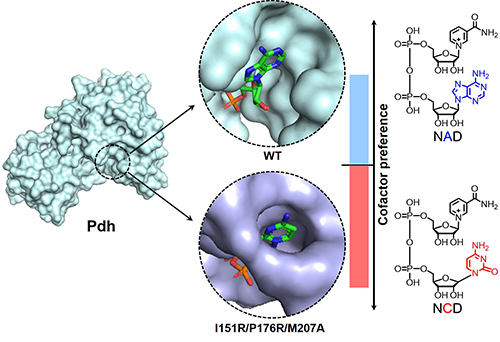
Structural information and cofactor preference for phosphite dehydrogenase and variant. (Image by LIU Yuxue and FENG Yanbin)
The natural redox cofactor nicotinamide adenosine dinucleotide (NAD) is omnipresent in biological systems and involved in complex processes. It is challenging to predict the consequences of NAD intervention, largely because redox cofactors are involved in diverse metabolism and processes.
To enable selective controlling over a specific redox biochemistry, Prof. ZHAO proposed an approach by implementing non-natural redox cofactors previously. He created bioorthogonal redox systems consisting of malic enzyme mutants that strongly favored NCD (J. Am. Chem. Soc.). NCD-linked metabolic circuits were successfully devised for reductive carboxylation of pyruvate into malate by using phosphite as the electron donor, and the system achieved higher malate yield from glucose by the engineered Escherichia coli cells (ACS Catal.).
In this work, the researchers further improved NCD preference of Pdh variants and collected X-ray crystal structures of 3 enzymes in their cofactor-free and cofactor-bound forms. It was found that the cofactor-binding pocket of Pdh became more crowded upon introduction of large and polar amino acid residues.
Further, these Pdh variants formed new molecular interactions to favor NCD binding. Such a paradigmatic study should promote efforts to engineer other enzymes to favor non-natural redox cofactors, and eventually bring them into life by chemical and synthetic biologists.
This work was supported by National Natural Science Foundation of China and DICP, and dedicated to the 70th anniversary of DICP, CAS. (Text by LIU Yuxue and FENG Yanbin)