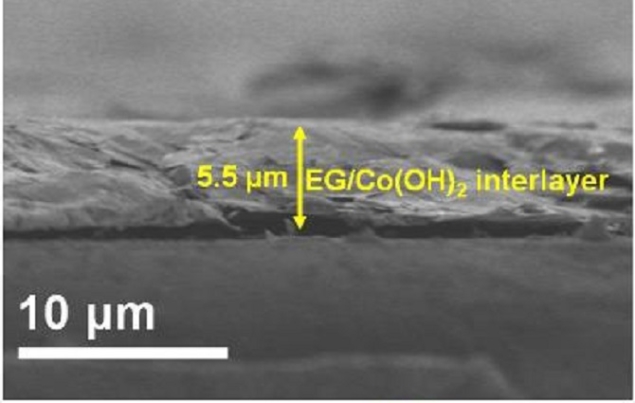
Shuttling stoppers: Cross-section scanning electron microscopy image of exfoliated graphene (EG)/Co(OH)2 interlayer/polypropylene(PP) separator. (Image by Journal of Physics: Energy)
Sulphur has recently proved a cheap and competitive partner for lithium ion batteries with an energy density of 2600 Wh/kg, in theory. In practice lithium-sulphur batteries have suffered from low cycling lifetimes attributed to the polysulphide ions shuttling to and from the electrodes during charge and discharge. Now researchers at the Dalian Institute of Chemical Sciences, the Chinese Academy of Science, have developed a hybrid interlayer combining graphene and Co(OH)2 nanosheets that prevents sulphide movement and enhances use of the sulphide ions to improve the battery capacity and cyclability.
Reporting their results in the first issue of the Journal of Physics: Energy Zhong-Shuai Wu and colleagues highlight the electrical insulation of elemental sulphur and the discharge products Li2S/Li2S2, as a challenge for optimizing performance, as well as the sulphide ion shuttling, which alongside volumetric expansion and lithium dendrite formation diminish the cycling lifetime.

Zhong-Shuai Wu (Image by physicsworld)
Interlayer innovations
Other groups have previously experimented with modifying the separator – a standard battery component between cathode and anode – with nonpolar carbon materials to prevent the sulphide ion shuttling. However, the interactions between sulphide ions and those modified separators were too weak to effectively block their diffusion. Wu and colleagues get around this with the hybrid interlayer they coat on the separator because the Co(OH)2 nanosheets are polar and so increase chemical interactions with the sulphides, while the graphene nanosheets serve as a physical barrier for preventing the diffusion and migration of the dissolved polysulfides. The high functionality of the graphene and Co(OH)2 nanosheets with a strong synergistic effect for suppressing the shuttle effect also allows low mass loading of just 0.20 mg cm-2.
“The EG [exfoliated graphene] sheets in hybrid interlayer can serve as a polysulfides physical barrier and electrical conductive network to increase the reuse of polysulfide trapped while the polar Co(OH)2 nanosheets can fulfil the role of chemical adsorption of polysulfides,” they explain in their report. “As a consequence, high-capacity Li-S batteries with 918 mAh/g at 0.5 C, accompanied with exceptional rate capability (677 mAh/g at 5 C) and stable cycle stability (565 mAh/g after 300 cycles at 0.5 C), are achieved.”
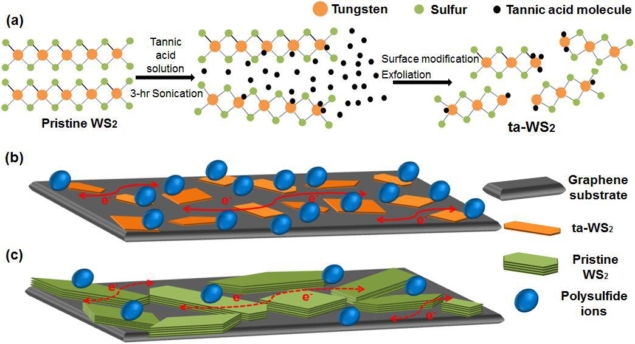
Tannic WS2 enhancements: (a) Schematic representation for effective exfoliation and modification of pristine WS2 with tannic acid assistant. (b) and (c) Schematic illustration of polysulfide species on the graphene mixed with ta-WS2 and pristine WS2, respectively. (Image by Journal of Physics: Energy)
Optimizing reaction rates
Da-Wei Wang at the University of New South Wales in Australia and Xin Tan at Australian National University and colleagues also report results of research focused on polysulphide batteries in the first issue of Journal of Physics: Energy. They suggest, “Although substantial effort has been devoted to the improvement of LIBs [lthium ion batteries] and various vigorous studies toward further development still continue, the present Li-ion technologies cannot fulfil the ever-increasing requirements of the modern society, because of the limited energy density and expensiveness in the large-scale applications.”
They add that the main barrier to uptake for polysulphide organic battery systems is the cost and flammability, which has prompted a lot of effort to make organic polysulphide systems that operate with an aqueous-based electrolyte. This has in turn led to frustrations with “sluggish” reaction rates. Wang and Tan and their colleagues tackle this with a conducting graphene support decorated with hydrophilic WS2 nanosheets exfoliated using tannic acid. In their report they highlight that as well as enabling efficient exfoliation of WS2 nanosheets, the tannic acid modifies them to enhance the hydrophilic properties thereby improving the electrocatalytic activity of the WS2 and boosting the battery reaction rates.
“The incorporation of tannic acid imposed the collective interactions between polysulfide and the WS2 nanosheets via the hydrophilic molecules and the polar surfaces,” they explain in their report. “With a 0.5 M Li2S2 electrolyte, the graphene and modified WS2 mixture gave an areal specific capacity of 0.37 mA h/cm2, compared to 0.27 mA h/cm2 for the pure graphene.”
Full details of both papers are available in the first issue of Journal of Physics: Energy. (physicsworld)