Finding may advance mission to make hydrogen fuel from water inexpensively
An easy-to-prepare catalyst consisting of isolated metal atoms embedded at various points across functionalized graphene sheets can carry out a key step in water splitting, according to a study (Nat. Catal. 2018, DOI: 10.1038/s41929-018-0158-6).
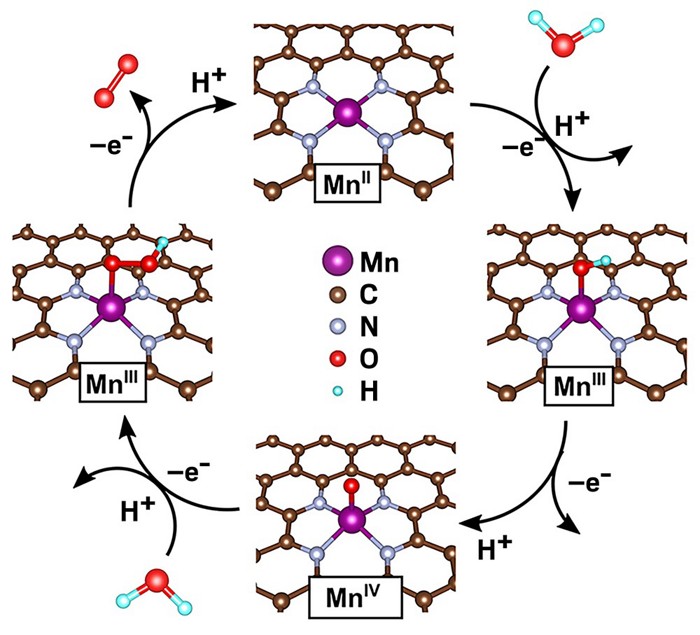
Graphene functionalized with isolated MnN4 units undergoes a catalytic cycle to convert water (starting at top right) to O2 (top left). (Image by Nat. Catal.)
If water could be split easily and inexpensively into molecular hydrogen (H2) and oxygen (O2), the world could draw a nearly limitless supply of clean-burning hydrogen fuel from the oceans. Inspired by nature’s use of a metal cluster (CaMn4O5) to generate O2 from water during photosynthesis, scientists have designed various multimetal-atom catalysts to facilitate the process, called the water oxidation reaction (WOR).
Several researchers have shown that various synthetic manganese-cluster catalysts actively mediate WOR. What remained unknown is whether a simpler, less expensive catalyst based on single manganese atoms rather than clusters can do the job actively and energy efficiently.
Yes, it can, concludes a team led by Can Li of the Dalian Institute of Chemical Physics. By reacting manganese chloride, graphene oxide, and ammonia, the researchers made a material in which isolated manganese atoms are embedded across a graphene surface, each surrounded by four nitrogen atoms. The team conducted water-oxidation tests to evaluate the material’s catalytic properties.
The researchers found that in contrast with pure graphene and nitrogen-doped graphene, which were inactive, their MnN4-graphene catalyst exhibited a WOR turnover frequency of up to 215 per second. Turnover frequency is a measure of catalytic activity that describes how many times a catalyst carries out a reaction in a given period. The MnN4-graphene value is nearly 100 times as high as that of other synthetic Mn-based catalysts and in the ballpark of naturally occurring ones. The team also found that the new catalyst mediates WOR at relatively low overpotential values, an indication of high energy efficiency.
A great deal of effort has been devoted to finding efficient, low-cost catalysts that drive water splitting, says Xiao Cheng Zeng of the University of Nebraska, Lincoln, who has studied the process computationally. “The water oxidation reaction is the most challenging step,” he says, so this experimental demonstration with manganese, which is much cheaper than precious-metal catalysts, “is a significant step in the right direction.” (c&en)