Recently, Prof. DEND Dehui from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences collaborated, along with Prof. WANG Ye and Prof. CHENG Jun from Xiamen University, directly synthesized ethylene glycol via selective C-C coupling of methanol. Their findings were published in Nature Communications.
The selective conversion of methanol into C2 or multi-carbon molecules via controlled C-C coupling is a challenging and attractive. Currently, the conversions of methanol involving C-C bond formation are restricted to carbonylation and dehydrative oligomerizations, such as processes of methanol-to-olefins (MTO) and methanol-to-aromatics (MTA), which show limited selectivity to a specific product.
“The preferential activation of inert C-H bond in methanol without affecting the hydroxyl group to produce ethylene glycol is one of the most challenge reactions in chemistry.” said Prof. DEND.
Currently, more than 90% of ethylene glycol is produced by petroleum-derived ethylene via the epoxidation and the subsequent hydrolysis of ethylene epoxide, leading to a low-efficient and high energy consumption process. The synthesis of ethylene glycol via dimethyl oxalate from coal-based syngas is a long technological and high-cost process.
The methanol can be derived from a variety of carbon resources, such as natural gas or shale gas, coal, biomass, and carbon dioxide. It is an abundant and renewable C1 building block. Therefore, the direct synthesis of ethylene glycol from methanol is of great significance.
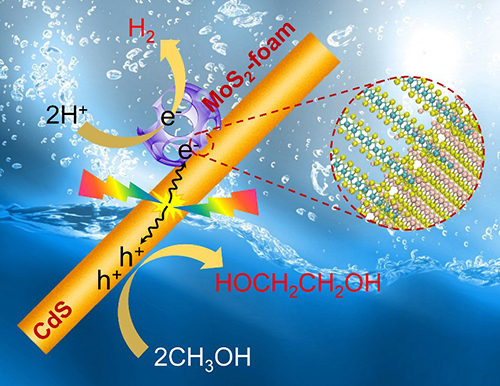
A schematic of direct conversion of methanol to ethylene glycol on the MoS2-foam/CdS catalyst. (Image by DENG Jiao Deng and XIE Shunji)
Based on the study of two-dimensional MoS2 catalytic materials (Nat. Commun., Nat. Nanotechnol., Energy Environ. Sci.). Scientists constructed a novel catalyst of mesoporous MoS2 nanofoam modified Cadmium Sulfide (CdS) nanorod, and achieved the direct synthesis of ethylene glycol via C-C coupling of methanol under visible light for the first time.
By designing a process-intensified reactor with ethylene glycol separation during the reaction, they could achieve a selectivity of 90%, a yield of 16% and a quantum efficiency (450 nm) of 5%.
The DFT calculations suggested that the cleavage of C-H bond in CH3OH on CdS surfaces occurred through a concerted proton-electron transfer (CPET) mechanism driven by the photogenerated hole, producing ·CH2OH radical with a low activation barrier. The weakly adsorbed ·CH2OH could readily desorb from CdS surfaces.
MoS2-foam could not only effectively promote the transfer of electrons and generate photogenerated holes on the surface of CdS, but also promoted the coupling of ·CH2OH into ethylene glycol in MoS2-foam channels.
The prepared MoS2-foam/CdS composite catalyst greatly promoted the formation rate of ethylene glycol, and the formation rate of ethylene glycol was over 20 times higher than that of pure CdS.
The present visible light-driven methanol conversion not only offers a high-efficient approach for ethylene glycol synthesis under moderate condition, but also opens up an avenue for designing new reactions, in particular for those involving preferential C-H bond activation without affecting other functional groups in the same molecule.
This work was supported by Ministry of Science and Technology of China, National Natural Science Foundation of China, Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, and Collaborative Innovation Center of Chemistry for Energy Materials (2011·iChEM). (Text by DENG Jiao and XIE Shunji)