Recently, Professor LI Gao from Gold Catalysis Research Center in Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) in collaboration with JIN Rongchao from Carnegie Mellon University (CMU) of USA successfully synthesized a chiral Ag nanocluster with special structure and optical properties. Their finding was published in Nature Communication.
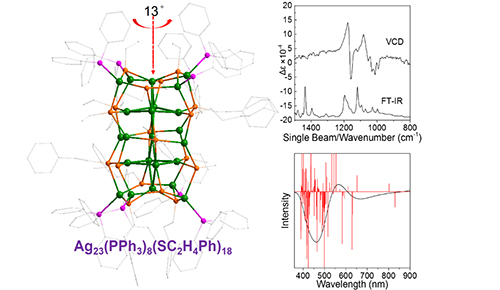
Total framework (left), FT-IR and VCD spectra (top-right), and simulated ECD spectrum (down-right) of the Ag23 nanocluster. (Image by LI Zhimin)
Chirality refers to the molecules that lack an internal plane of symmetry, and hence are non-super imposable with their mirror image. Chiral noble metal nanostructures, in particular silver and gold, exhibit characteristic localized surface plasmon resonance, which leads to an intense optical activity.
Chiral metal nanoparticles can also serve as a new route to enantioselective catalysis owing to their capability of enantioselective adsorption of chiral compounds, which is a key step toward achieving enantiospecific catalysis, separation, and sensing.
To date, the direct and one-pot synthesis of chiral nanoclusters with an intrinsically chiral metal core, which uses metal ion and achiral ligands as the precursors only, is an attractive challenge.
“Our work is the first case of direct synthesis of chiral nanoclusters with an intrinsically chiral core capped by achiral ligands.” said Prof. Li.
Scientists achieved the Ag23(PPh3)8(SC2H4Ph)18 nanocluster via a simple reduction of silver nitrate. They used sodium borohydride in the presence of excess triphenyl phosphine and phenylethanethiol.
The Ag kernel structure was composed of two face-centered cubic Ag14 units with a around 13 degree twist, resulting in the properties of helicity and chirality.
A combination of vibrational circular dichroism spectroscopies as well as DFT (density functional theory) simulations, were applied to investigate the cluster’s chirality. Their findings clearly showed that the twist-induced chirality of the Ag23 cluster and the excitation of paired electrons were mainly responsible for the main absorption band, which were in good agreement with experimental results.
The research was supported by National Natural Science Foundation of China. (Text by LI Zhimin)