The topic of Lithium-Sulfur (Li-S) batteries has become one of the hottest international research spots due to the high energy density and the low cost properties of Li-S batteries. Currently, the shuttle effect of polysulfide and the unstable interface of lithium anode are the key issues, which obstruct the development of Li-S batteries.
For a long time, scientists are used to utilize LiNO3 additive to solve the polysulfide shuttle problem in Li-S battery. However, it also brings in potential safety hazard due to nitrate, carbon and sulfur coexistence in battery system.
To solve this problem, the research group of Energy Storage Technology Research Department, which is led by ZHANG Huamin, LI Xianfeng and ZHANG Hongzhang from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), designed a new LiNO3-free electrolyte.
This novel electrolyte has low solubility (Ksp) of polysulfide as well as stable SEI film on lithium, which enabled high stability, safety and capacity output of Li-S batteries. And the results were published in Nano Energy, 39, 262–272.
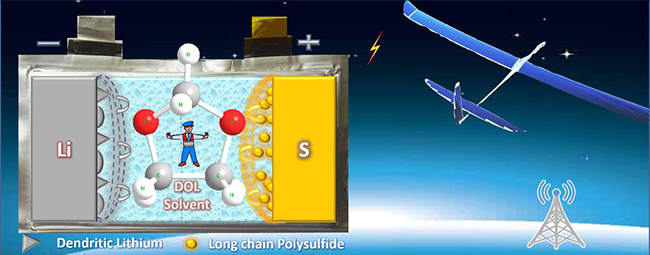
LiNO3-free electrolyte with low solubility of polysulfide and low dendrite of lithium for Li-S battery(Image by QU Chao and CHEN Yuqing)
This is the firstly reported LiNO3–free electrolyte with high-performance: Low Ksp of polysulfide, high conductivity, high active materials utilization, and excellent anode interface stability.
Scientists have tried to assemble 4000 mAh soft package Li-S battery with this electrolyte. And it could realize the high energy density of 350 Wh/kg, the high power density of 60 W/kg, and 30-cycled stable circulation. Such battery is expected to make solar unmanned aircraft flight one month in a row.
Meanwhile, this work provides a new idea for design and preparation of Li-S batteries electrolyte.
The above research work was supported by the National Natural Science Foundation of China, the Collaborative Innovation Centre of Chemistry for Energy Materials of the Ministry of Education (iChEM), the Youth Innovation Promotion Association of the Chinese Academy of Sciences and the Youth Innovation Foundation of Dalian Institute of Chemical Physics. (Text by QU Chao and CHEN Yuqing)
Dr. WANG Yongjin
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84374221
E-mail: wangyj@dicp.ac.cn