The electrochemical CO2 reduction reaction (CO2RR) is powered by renewable electricity or surplus nuclear electricity. It can be used to prepare carbon monoxide, formic acid, hydrocarbon, alcohol, other high value fuel and chemicals in one step under relatively mild reaction conditions. The process can simultaneously achieve efficient CO2 conversion and clean energy storage. At present, designing efficient catalysts to decrease the overpotential and enhance reaction selectivity is the challenging research focus for CO2RR.
Prof. BAO Xinhe's research team in the State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, has recently made research progress in metal-oxide interface enhanced CO2 electroreduction.
Based on the previous CO2RR studies and the understanding of the metal-oxide interfacial confinement catalysis, they design and prepare carbon supported Au-CeOx catalyst with metal-oxide interface structure. They also investigate the relationship between the Au-CeOx interface and catalytic performance of CO2RR. The CO Faradaic Efficiency reaches 89.1% over Au-CeOx/C at -0.89 V vs. reversible hydrogen electrode (RHE). The result is significantly higher than 59.0% and 9.8% over Au/C and CeOx/C at the same potential. The CO geometric current density over Au-CeOx/C (12.9 mA cm-2) is about 1.6 times of that over Au/C (8.3 mA cm-2) at -0.89 V vs. RHE. In situ scanning tunnelling microscopy and synchrotron-radiation photoemission spectroscopy show that the Au-CeOx interface is dominant in enhancing CO2 adsorption and activation. It can be further promoted by the presence of hydroxyl groups. Density functional theory calculations indicate that the Au-CeOx interface is the active site for CO2 activation and the reduction to CO. The synergy between Au and CeOx promotes the stability of key carboxyl intermediate (*COOH), thus facilitates CO2RR. Similar interface-enhanced CO2RR is further observed on Ag-CeOx, demonstrating the generality of the strategy for enhancing CO2RR. The research results may provide a new way to regulate the CO2RR performance, and enrich and expand the concept of nano-confined catalysis proposed by the research team.
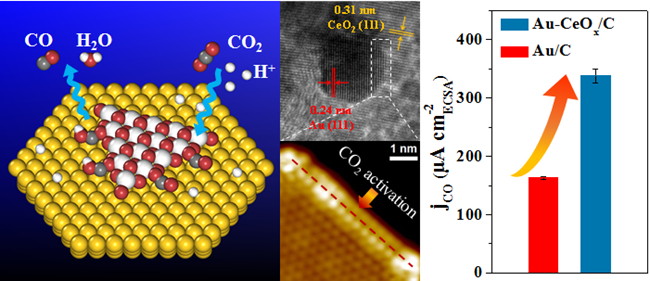
Enhancing CO2 Electroreduction with the Metal-Oxide Interface(Image by GAO Dunfeng, ZHANG Yi and ZHOU Zhiwen)
The results are published in Journal of the American Chemical Society (J. Am. Chem. Soc. 2017, 139, 5652). This work was financially supported by Natural Science Foundation of China, National Key R&D Program of China and Strategic Priority Research Program of the Chinese Academy of Sciences. (Text and Image by GAO Dunfeng, ZHANG Yi and ZHOU Zhiwen)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201
E-mail: luxinyi@dicp.ac.cn