Converting CO2 from a detrimental greenhouse gas into value-added liquid fuels not only contributes to mitigating CO2 emissions, but also reduces dependence on petrochemicals. However, since CO2 is a fully oxidized, thermodynamically stable and chemically inert molecule, the activation of CO2 and its hydrogenation to hydrocarbons or other alcohols are challenging tasks. Most research to date, not surprisingly, is focusing on selective hydrogenation of CO2 to short-chain products, while few studies on long-chain hydrocarbons, such as gasoline-range (C5–C11) hydrocarbons. The key to this process is to search for a high efficient catalyst.
CO2 Hydrogenation to Gasoline-range Hydrocarbons Over Na–Fe3O4/Zeolite Multifunctional Catalyst
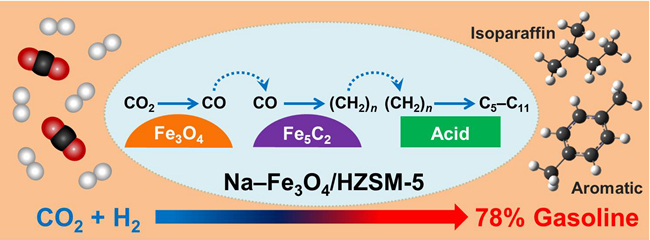
The research team led by Dr. SUN Jian and Prof. GE Qingjie in Dalian Institute of Chemical Physics, has succeeded in preparing a high efficient, stable, and multifunctional Na–Fe3O4/HZSM-5 catalyst for the direct production of gasoline from CO2 hydrogenation. This catalyst exhibited 78% selectivity to C5–C11 as well as low CH4 and CO selectivity under industrial relevant conditions. And gasoline fraction are mainly isoparaffins and aromatics thus favouring the octane number. Moreover, the multifunctional catalyst exhibited a remarkable stability for 1,000 h on stream, which definitely has the potential to be a promising industrial catalyst for CO2 utilization to liquid fuels.
In-depth characterizations indicate that this catalyst enables RWGS over Fe3O4 sites, olefin synthesis over Fe5C2 sites and oligomerization/aromatization/isomerization over zeolite acid sites. The concerted action of the active sites calls for precise control of their structures and proximity. This study paves a new path for the synthesis of liquid fuels by utilizing CO2 and H2. Furthermore, it provides an important approach for dealing with the intermittency of renewable sources (sun, wind and so on) by storing energy in liquid fuels.
This work was recently published on Nature Communications (DOI: 10.1038/ncomms15174). This work was financially supported by the National Natural Science Foundation of China, and the Hundred-Talent Program of DICP, Chinese Academy of Sciences. (Text and Image by WEI Jian)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201
E-mail: luxinyi@dicp.ac.cn
Embargo
1000 London time (BST) / 0500 US Eastern Daylight Time
1800 Japanese time / 1900 Australian Eastern Time