To find a suitable material to store hydrogen under moderate temperature and hydrogen pressure is a key roadblock towards the commercialization of hydrogen powered fuel cell vehicles. In the recent years, many hydrides of light elements (HLEs) have attracted considerable attention for use as on-board hydrogen storage materials due to their high volumetric and gravimetric hydrogen densities.
However, their application is limited by severe hydrogenation/de-hydrogenation kinetic barriers and poor intrinsic thermodynamics. Although several approaches have been reported to improve the material thermodynamic and kinetic properties, a material, which can fully satisfy the requirements such as fully reversible and operating near ambient temperature set by the automotive industry, was not found yet.
Dalian Institute of Chemical Physics (DICP) group led by Prof. CHEN Ping demonstrates that the synergy among LiBH4, Mg(NH2)2 and LiH, three of the most-investigated HLEs, can lead to a fully reversible hydrogenation and dehydrogenation cycle at temperatures below 373 K. The measured dehydrogenation enthalpy of this intriguing material is only 24 kJ(mol-H2)-1, which theoretically allows to de-hydrogenate the material at 273 K and 3.2 bar H2. Furthermore, experimentally it was shown that full H2 absorption can be achieved at 326 K and de-hydrogenation at 371 K, the lowest operating temperatures for hydrides of light elements as reported before.
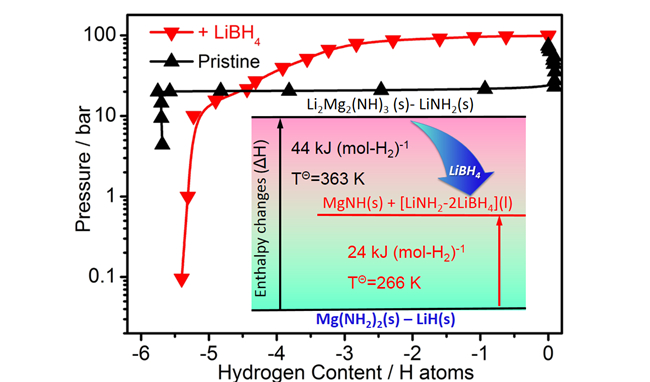
The Pressure-Composition-Temperature desorption curves of +LiBH4 and Pristine (Image by WANG Han)
The DICP researchers attribute the exceptional material properties to a possible “solvent” like behavior of LiBH4 in stabilizing the intermediates and products of the hydrogenation/de-hydrogenation cycle. Such an understanding of the role of LiBH4 can devise design and development of multi-component HLEs systems in which one or multiple components may serve as a medium to modulate the thermodynamic and kinetic properties of hydrogenation and dehydrogenation.
This work was published on Advanced Energy Materials (DOI: 10.1002/aenm.201602456). This work is financially supported National Science Funds for Distinguished Young Scholars, the Collaborative Innovation Center of Chemistry for Energy Materials (2011-iChEM), CAS-Helmholtz JRG Project and National Natural Science Foundation of China. (Text and Image by WANG Han)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201
E-mail: luxinyi@dicp.ac.cn