Ortho-Quinone methides (o-QMs) are important intermediates in organic synthesis and materials chemistry as well as biological processes. However, the asymmetric reactions with o-QMs generated in situ are unexplored.
The Molecular Modeling and Design Group from Dalian Institute of Chemical Physics (DICP), Chinese Academy of Sciences has developed a highly efficient protocol for the preparation of optically active benzylic sulfones and 4-substituted chromans. This work has been published recently as a communication in Angew. Chem. Int. Ed. (DOI:10.1002/anie.201700250)
The Molecular Modeling and Design Group has been working on developing new heterogeneous methodolgies for a long time (Angew. Chem. Int. Ed., 2002, 21; Angew. Chem. Int. Ed., 2007, 6861), especially on the water-oil biphases system which has been widely used in a series of asymmetric catalytic reactions (Chem. Eur. J., 2004, 2277; J. Catal., 2007, 360; Green Chem., 2011, 1983; Angew. Chem. Int. Ed., 2012, 13159). In the previous work, this group realized the first conjugate addition of trityl thiol to in situ generated o-QMs. It was catalyzed by a chiral organic base with excellent enantioselectivities by using the advantage of water-oil biphases catalytic system. It consist of spatial separation between the organic-base catalyst, reactants in the organic phase, and the inorganic base in the aqueous phase, thus to suppress the racemic background reaction.
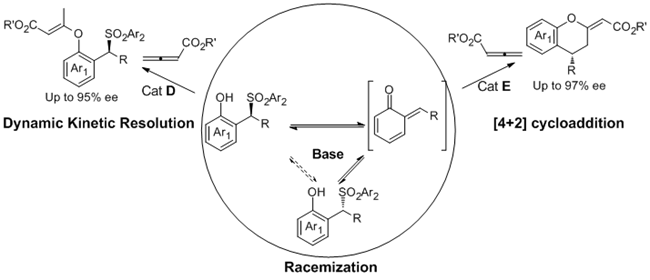
Different reaction pathways of 2-sulfonylalkyl phenols with allenic esters via o-QM intermediates: DKR versus [4+2] cycloaddition. (Image by LIU Yan and CHEN Ping)
In this work, it was found the strong base Et3N can precede the racemization of the o-QMs precursor. So that it can afford the possibility for the design of dynamic kinetic resolution (DKR) involving the high reactive intermediates. This group reported the first DKR involving o-QMs intermediates. In the presence of Et3N and cinchonine-derived nucleophilic catalyst, the DKR of 2-sulfonylalkyl phenols with allenic esters afforded chiral benzylic sulfones with good to excellent enantioselectivities (85-95% ee). Furthermore, using 2-(Tosylmethyl) sesamols or 2-(Tosylmethyl) naphthols which can generate stable o-QMs as the substrates, a formal [4+2] cycloaddition was achieved, delivering 4-aryl- or alkyl-substituted chromans with excellent enantioselectivities (88-97% ee). This work also provided a new strategy to design new asymmetric reactions involving o-QMs.
The first author of this work is PhD candidate CHEN Ping, co-supervised by Prof. LIU Yan and Prof. LI Can. The research work was financially supported by the National Natural Science Foundation of China and the Strategic Priority Research Program of the Chinese Academy of Sciences. (Text and Image by LIU Yan and CHEN Ping)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201
E-mail: luxinyi@dicp.ac.cn