Functionalized aromatic amines are important industrial intermediates for pharmaceuticals, agrochemicals, fine chemicals, dyes and polymers. They were generally synthesized by chemoselective hydrogenation of the corresponding functionalized nitroarenes. However, the traditional noble-metal catalysts were active not only for the reduction of the nitro group but also for the hydrogenation of other unsaturated functional groups existing in the same molecule.
Prof. ZHANG Tao's group in Dalian Institute of Chemical Physics has synthesized a gold catalyst for chemoselective hydrogenation of functionalized nitroarenes. By using the Zinc-Aluminum hydrotalcite (ZnAl-HT) supported thiolated Au25 nanoclusters (NCs) as the precursor of the catalyst, they prepared the high selectivity Au catalyst for the selective hydrogenation of functionalized nitroarenes in wide temperature and time ranges.
Previously, Prof. ZHANG's group have developed a rationally designed FeOx supported Pt single-atom and pseudo single-atom catalyst for the hydrogenation of functionalized nitro groups. By precisely controlling the reaction time and temperature, they obtained the good activity and selectivity for this kind of reaction. But the nitro group and the other unsaturated groups were competitively adsorbed on the catalyst so that excessive hydrogenation was inevitable while the concentration of the nitro group decreased.
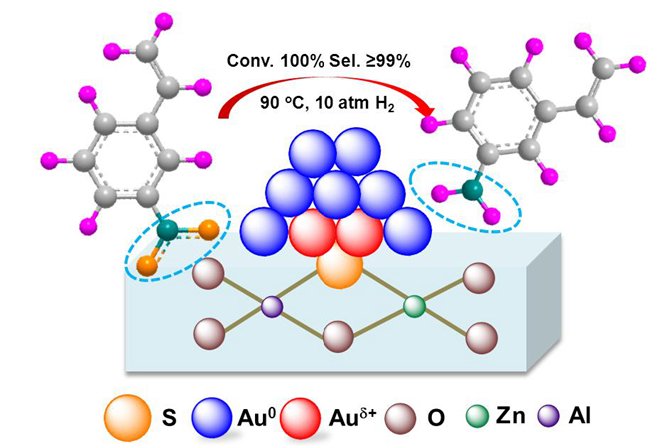
Figure 1. The reaction model of selective hydrogenation of 3-nitrostyrene over the Au25/ZnAl-HT-300 catalyst.(Image by TAN Yuan and LIU Xiaoyan)
Au catalyst exhibits high selectivity for various oxidation and hydrogenation reactions, due to the weak adsorption of reactant and product molecular. It has becoming one of the most important research projects in Prof. ZHANG's group in the last decade. They have done a series of important work on this topic. (Nano Today, 2013, 8, 403-416; J. Catal., 2013, 308, 258-271, 50th Anniversary Special Issue). This time they prepared a gold catalyst with high selectivity for the hydrogenation of functionalized nitroarenes to the target product.
Compared with traditional gold catalysts, the thiolated Au25 NCs supported on the ZnAl-hydrotalcite was highly resistant to sintering, with well controlled size (around 2.0 nm) even after calcination at 500 oC. The formation of the Au-S-Zn bond and the epitaxial interaction between gold and the support might contribute to the stabilization of the gold nanoclusters. Besides, the results of the control experiments suggested the catalyst was inert to adsorb other unsaturated groups, thus made it as a potential candidate for selective hydrogenation reactions. It is quite different from the behavior of the supported platinium-group metals.
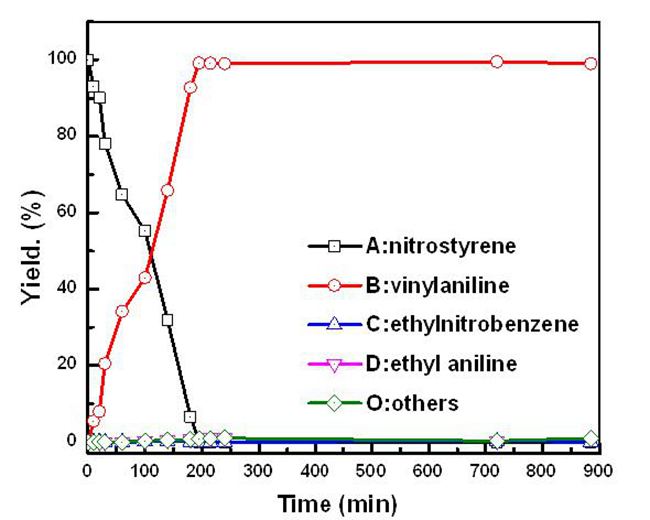
Figure 2. The evolution of the product distribution of the chemoselective hydrogenation reaction of 3-nitrostyrene over the Au25/ZnAl-HT-300 catalyst.(Image by TAN Yuan and LIU Xiaoyan)
This work was published on Angew. Chem. Int. Ed..The research work was financially supported by the National Natural Science Foundation of China, the National Key Projects for Fundamental Research and Development of China, the Youth Innovation Promotion Association CAS, and the Strategic Priority Research Program of the Chinese Academy of Sciences. The work was also supported by the BL 14W at the Shanghai Synchrotron Radiation Facility (SSRF) for the XAFS experiments.(Text and Image by TAN Yuan and LIU Xiaoyan)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201
E-mail: luxinyi@dicp.ac.cn