As an important class of human proteins, enzymes catalyzed more than 6000 reactions in human body and played very important roles in both exogenous and endogenous metabolism. Thus, it is recognized as key factors affecting human physiological and pathological processes. It is urgently necessary to develop a practical strategy to guide the rational design of isoform specific probes for a target enzyme.
Cytochrome P450 1A (CYP1A) is a membrane-bound enzyme, which is widely distributed in the hepatic and extrahepatic tissues. It consists of two major isoforms including CYP1A1 and its homology CYP1A2. Based on the subtle differences in 3D structure and substrate preference between CYP1A1 and CYP1A2, CYP1A1-specific probe “NBCeN” was designed by modifying the metabolic site of known CYP1A substrate. With the aid of both molecular docking-based virtual screening and reaction phenotyping-based experimental screening, the world’s first CYP1A1-specific probe was synthesized.
The newly developed fluorescent probe exhibited high selectivity towards CYP1A1 rather than other CYP enzyme, which could be distinguished between CYP1A1 and CYP1A2. Meanwhile, NBCeN could be combined used in both basic researches and clinical applications. For example, it can real-time monitor the enzyme activity of CYP1A1 or CYP1A2 in complex biological systems and rapid screen inhibitors or inducers of CYP1A1 or CYP1A2. It also can be used for imaging of intracellular CYP1A or CYP1A1 in living cells and tissues. Furthermore, the strategies used in this study will be very helpful for the design, screening and optimization of isoform specific probe substrates for other target enzyme.
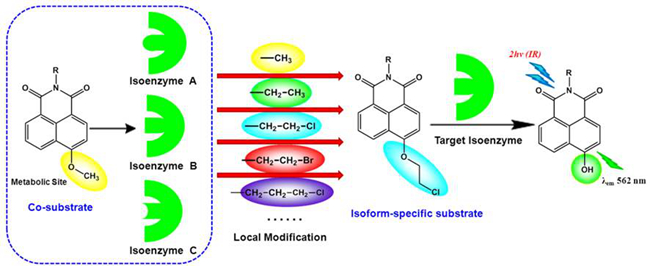
A practical strategy to develop an isoform-specific fluorescent probe for a target enzyme via local modification at the metabolic site (Imaged by DAI Ziru)
As early as 2015, Pharmaceutical Resource Discovery Group in Dalian Institute of Chemical Physics has designed another highly selective probe NCMN for human cytochrome P450 1A via modification at a site far way of the metabolic site. (J. Am. Chem. Soc.,2015, 137: 14488–95)
The related results have been published in Chemical Science (DOI: 10.1039/C6SC03970G). This work was supported by National Natural Science Foundation of China, National Basic Research Program of China and State Key Laboratory of Fine Chemicals. (Text and Imaged by DAI Ziru)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201,
E-mail: luxinyi@dicp.ac.cn