Numerous substrates could be identified when mass spectrometry-based proteomics is used to monitor the enzymatic reactions occurred in complex system with the presence of thousands of substrates. However, prioritizing new substrates is usually not achieved because the lacking of validated kinetic theory for such a complex system.
The research team headed by Prof. ZOU Hanfa and YE Mingliang from Dalian Institute of Chemical Physics (DICP) developed and validated kinetics models to facilitate understanding of enzyme kinetics in complex system on the basis of previous work. Especially, the ratio of substrate depletion was found to be independent of other coexisted substrates when the total substrate concentration is relative low. Based on this, the specificity constants, the enzymological constants reflecting the relative rates of an enzyme acting on different substrates, of numerous protease substrates presented in the complex system were accurately determined.
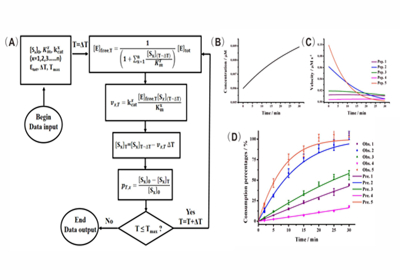
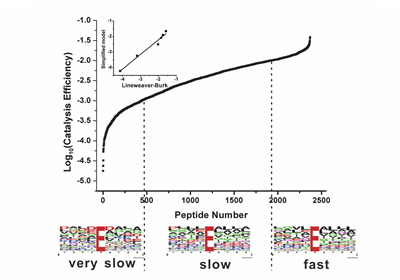
Enzyme specificity is fundamental in physiology, as metabolism would be impossible if enzymes could not distinguish between strictly similar substrates, and all reactions proceeded unchecked toward thermodynamic equilibrium. A significant contribution of this study is that it provides a theory to determine the enzyme specificity in high throughput. This work is published as an article in Molecular & Cellular Proteomics (doi:10.1074/mcp.M116.062869).(Text and Imaged by DENG Zhenzhen)