The hydrogen separation and purification technology is significantly important for the applications of hydrogen in various fields. Compared to other methods for hydrogen separation, inorganic dense hydrogen-permeable membranes exhibit 100% permeation selectivity for hydrogen. So they are regarded as one of the most promising methods for high-purity hydrogen production. However, the state-of-art Pd-based metallic membranes are limited by the scarce resource of Pd and low stability under H2S-containing atmosphere. In addition, another type of inorganic dense hydrogen-permeable membranes, i.e. proton conducting membranes, exhibit low hydrogen permeability and are also easily poisoned by H2S.
To solve above problems, Prof. YANG Weishen and Prof. ZHU Xuefeng from State Key Laboratory of Catalysis in Dalian Institute of Chemical Physics developed a new method for hydrogen separation by using an oxygen-permeable ceramic membrane. It's successfully demonstrated by experiments with excellent performance. The hydrogen separation rate is high up to 16.3 mL·cm-2·min-1 with separation factor up to >10,000. This separation rate is 2-3 magnitudes higher than the proton conducting membranes, and comparable to Pd-based metallic membranes. Besides, no performance degradation was observed in a long-term operation with the feeding 200 ppm H2S containing gas. The experiment results indicate that the membranes exhibit high selectivity, high hydrogen separation rate and stability under H2S-containing atmosphere. It could be used to produce high-purity or ultra-high-purity hydrogen for fuel cells, semiconductor manufacturing, photovoltaic cells production, etc.
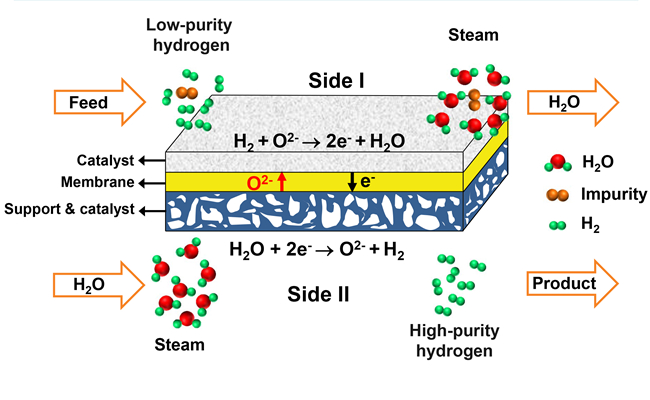
The concept of hydrogen separation by using the oxygen-permeable ceramic membrane (Imaged by LI Wenping)
The figure shows the concept of hydrogen separation by using the oxygen-permeable ceramic membrane. The low-purity hydrogen is fed to one side of the membrane (Side I) and the steam is fed to the other side of the membrane (Side II). On the Side II, water is spited into hydrogen and oxygen ions at elevated temperature. And then oxygen ions diffuse to the Side I and react with hydrogen to produce water. Therefore, the high-purity hydrogen is acquired after condensation and drying of the outlet gas from Side II. The amount of hydrogen produced from Side II is equivalent to that consumed on Side I. As a whole, there is no net chemical reaction occurring, but a mass exchange is achieved between the two sides. Thus, it is reasonable to regard this process as a hydrogen separation process with the help of steam.
The related results were published in Energy Environ. Sci. (DOI: 10.1039/C6EE02967A). This work was supported by Natural Science Foundation of China,Chinese Academy of Sciences and DICP DMTO project. (Text and Imaged by LI Wenping)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201,
E-mail: luxinyi@dicp.ac.cn