A review on catalytic C?alkylation of ketones and related compounds by alcohols through borrowing hydrogen strategy was recently published in Angew. Chem. Int. Ed. (2016, 55, 862-875) by Prof. Zhengkun Yu, et al. from the Laboratories of Organometallic Catalysis and Synthesis, Chinese Academy of Sciences (http://onlinelibrary.wiley.com/doi/10.1002/anie.201507521/full).
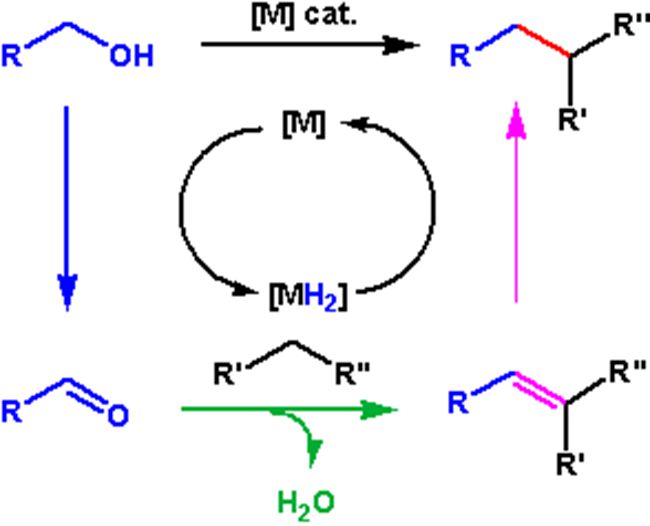
Highlight on C?Alkylation of Ketones and Related Compounds by Alcohols Through Borrowing Hydrogen Strategy(Photo by Ping WU)
Transition metal-catalyzed C-alkylation of ketones and secondary alcohols with alcohols avoids use of organometallic or environmentally unbenign alkylating agents by means of borrowing hydrogen (BH) activation of the alcohol substrates. Water is formed as the only by-product, which makes the BH process atom-economical and environmentally benign. Diverse homogeneous and heterogeneous transition metal catalysts, ketones, and alcohols can be used for such a purpose, rendering a BH process to be promising for replacing the traditional procedures using alkylating agents in organic synthesis. This minireview summarizes the advances during the last five years in transition metal-catalyzed BH ?-alkylation of ketones, and ?-alkylation of secondary alcohols with alcohols. Discussion on the application of the BH strategy for C-C bond formation is given. (Text/Photo by Ping WU)