Recently, Professor Jie Xu and Professor Fang Lu of organic catalysis group have developed a novel synthetic route to directly obtain diethyl terephthalate (DET) from biomass-derived muconic acid through a cascade process combining esterification, Diels-Alder cycloaddition, and dehydrogenation reaction. This work is now online published in the journal of Angewandte Chemie International Edition as hot paper (Angew. Chem. Int. Ed. 2016, 55, 249-253).
The annual nameplate capacity of purified terephthalic acid (PTA), a large-volume commodity chemical used to produce polyesters (PET, PTT and PBT), was over 46 million tons in China, 2014. Currently, industrial production of PTA is mainly through the oxidation of petroleum-based para-xylene (PX), and Xu’s group has a long-term research accumulation in the oxidation route for the production of PTA. This study has important scientific significance and application prospects to utilize renewable biomass resources to synthesis PTA and its derivatives.
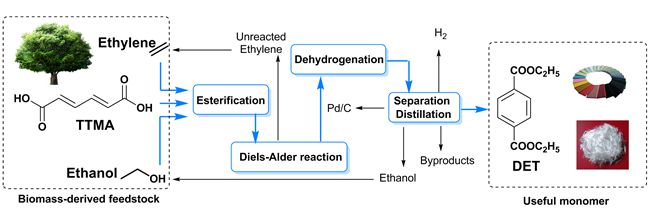
Muconic acid can be produced from glucose and lignin by microbial synthesis. It contains a conjugated diene and two carboxylic acid moieties, which render it an attractive candidate for the synthesis of PTA. However, the solubility of muconic acid is very poor in many solvents, and its molecular structure contains two electron-withdrawing carboxylic acid groups, rendering its reaction with a dienophile difficult. To solve these problems, a synthetic route to diethyl terephthalate (DET), using muconic acid, ethanol, and ethylene as the reactants, through a cascade process combining esterification, Diels–Alder cycloaddition, and dehydrogenation has been proposed. The key esterification reaction improves the solubility of the reaction products in ethanol and modulates the electronic properties of muconic acid, thus promoting the Diels–Alder reaction with ethylene. The total yield of diethyl terephthalate reached 80.6% based on the amount of muconic acid used in the two-step synthetic process. This work provides an example of producing high-value aromatic chemicals by fully utilizing the existing specific structures of biomass-derived feedstock. By rational design of the functional groups, the utilization of biomass can thus become more economic and environmentally friendly.
This work was financially supported by the National Natural Science Foundation of China. (Text/Photo by Rui LU and Weiqiang YU)