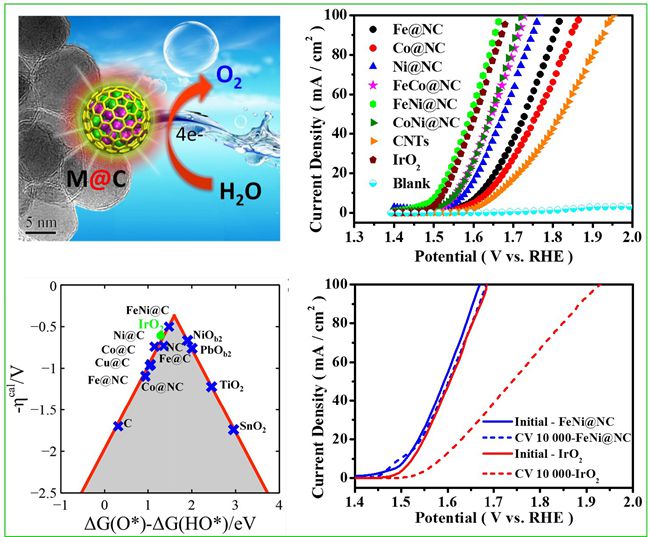
Hydrogen, as a clean, efficient and renewable energy, attracts great attention in recent years. The production of hydrogen through electricity-driven water splitting is an important method to realize the low-cost industrialization. The oxygen evolution reaction (OER), as a key half-reaction involved in water splitting, is kinetically sluggish and largely hinder the overall efficiency of water splitting. Current commercial OER catalysts still rely on the use of ruthenium (Ru) and iridium (Ir) oxides, but they are not suitable for large-scale applications because of their scarcity and high cost. Consequently, tremendous research efforts have been devoted to developing low-cost and earth-abundant non-precious-metal catalysts. Recently, on the basis of previous studies towards nano-carbon catalysis, associate Prof. Dehui Deng and Prof. Xinhe Bao etc. from SKLC, DICP have innovated the preparation strategy to directly synthesize single layer graphene encapsulating uniform earth-abundant 3d transition metal nanoparticles, such as Fe, Co, Ni and their alloys via a chemical vapor deposition (CVD) process in a confined channel of mesoporous silica (SBA-15). The single layer graphene encapsulating FeNi alloy (FeNi@NC) showed excellent OER activity and high durability, which even exceed that of commercial IrO2 catalyst, showing great potential to replace precious metal catalysts in OER. This work has recently been published as a communication in Energy & Environmental Science (Energy Environ. Sci., 2016, DOI: 10.1039/C5EE03316K).
DICP Researchers Innovate the Preparation Strategy to Directly Synthesize Single Layer Graphene Encapsulating Uniform Earth-abundant 3D Transition Metal Nanoparticles(Photo by Ying Shi and Xiaoju Cui)
DFT calculations and experiments indicate that the single atomic thickness of graphene shell immensely promotes the electron transfering from the encapsulated metals to the graphene surface, which efficiently optimizes the electronic structure of the graphene surface and therefore triggers the OER activity of the inert graphene surface. Also, the graphene shell could efficiently prevent the corrosion of 3d TMs from the harsh environment. The concept of “electron penetration”, which describes the above mentioned catalytic process, was first proposed in 2013 by this group (Angew. Chem. Int. Ed. 2013, 52, 371). It has been widely accepted by the international counterparts and described vividly as “chainmail for catalyst”. In recent years, this group has made several important progress towards this concept, e.g. they found that the thickness of graphene shells (chainmail) can remarkably affect the electron penetration process, the catalytic oxygen reduction activity (J. Mater. Chem. A 2013, 1, 14868) and hydrogen evolution reaction (HER) activity (Angew. Chem. Int. Ed. 2015, 54, 2100) by experiments and DFT calculations. Besides, they proposed the HER mechanism on the graphene surface (Energy Environ. Sci. 2014, 7, 1919). They also collaborated with other research group to study the graphene shell encapsulating FeNi catalyst as the counter electrode material of dye-sensitized solar cells and found that the catalyst showed superior activity than the precious Pt in the reduction of I3- (Angew. Chem. Int. Ed. 2014, 53, 7023). In addition, with the aid of soft X-ray imaging technique, the electronic structure of the graphene surface modulated by the active metals was directly observed (Chem. Sci., 2015, 6, 3262). Based on the experimental data and theoretical calculations, the nature of the interaction between graphene surface and 3d metals was illustrated deeply, and a relatively complete concept was gradually formed.
These works are supported by National Natural Science Foundation of China, Strategic Priority Research Program of the Chinese Academy of Sciences, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM). (by Ying Shi and Xiaoju Cui)