Solar hydrogen production via a photocatalytic process has been drawing great attention in recent years due to the increasing concerns on energy and environmental problems. The requirement for a photocatalytic reaction on semiconductor-based photocatalyst is that the energy of incident light is larger than the band gap of the photocatalyst (Eλ Eg), and then electrons and holes can be generated and separated for reduction and oxidation reactions, respectively. Until now, almost all the reported photocatalysts are semiconductor-based materials with a suitable band structure, but none of the insulators can be used for photocatalytic hydrogen production because their band gaps are too large to be excited by the usual UV and visible light sources.
Recently, a new progress about the photocatalytic hydrogen production on an insulator has been made by the Solar Energy Division (DNL 16) at DICP, led by Prof. Can Li. The work has been recently published in《Scientific Reports》(Rengui Li and Can Li et al, Sci. Rep., 2015, 5, 13475; http://www.nature.com/articles/srep13475).
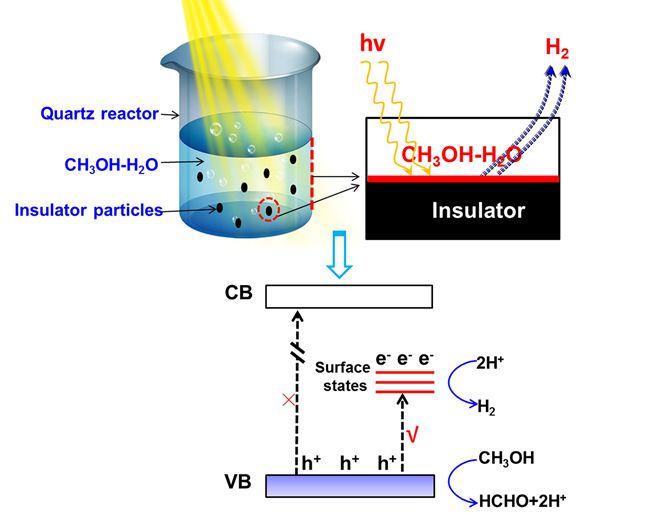
Using CH3OH molecule as a hole scavenger, they found that a considerable amount of H2 can be generated at a quartz surface using light with energy far outside the electronic absorbance range of the CH3OH-H2O solution and the excitation energy of the quartz. This particular H2 production was further confirmed using two different lasers (266 nm and 355 nm) as light sources. Interesting, the results showed that the incident light with a wavelength up to 400 nm can even induce H2 production from the CH3OH-H2O solution at quartz surface. It was generally believed that CH3OH itself cannot contribute to H2 production because it does not absorb any light from commonly used light sources, so this process should not occur in principle via either conventional photocatalysis or a photochemical process. They found that when some insulator oxide (SiO2 or Al2O3) particles onto which Pt has been deposited as reduction cocatalyst were added to the reaction solution, the H2 production can be strongly enhanced, conforming that the H2 was produced from the reduction of protons by the photogenerated electrons.
The Photo-induced Hydrogen Production Can Take Place at An Insulator Surface(Photo by LI Rengui)
Further characterizations by photoluminescence (PL) and electron spin resonance (ESR) technology suggested that there are electrons present in the defect states of quartz, which could be excited from the valence band of insulators (e.g., quartz reactor, SiO2 or Al2O3), which could be excited by the light with the incident energy smaller than its bandgap. The traditional quartz is made of SiO2 that is calcined at high temperatures (usually over 700 °C), and some defect sites could be unavoidable generated on the quartz surface after calcination at high temperatures, and these defect sites can act as electron acceptors, making excitation from the valance band of quartz to these states possible. This process will be responsible for the H2 production via electron-proton coupling from the CH3OH-H2O solution at the insulator surface. As far as we know, this example is the first to show that H2 production can be achieved at an insulator surface, demonstrating the possibility of photo-induced H2 production on insulators. Our work demonstrates that photo-induced H2 production can take place on insulator surfaces, which are commonly believed to be inert, and will shed light on the surface nature of insulators.
This work was financially supported by NSFC and MOST 973 program. (Text/ Photo by Li Rengui)