Recently, Dr. Yanqiang Huang from the State Key Laboratory of Catalysis led by Prof. Tao Zhang, in collaboration with Dr. Xinkui Wang from Dalian University of Technology, has made new progress in gold catalysis. They were the first to realize pure formic acid dehydrogenation by using a heterogeneous Schiff base functionalized gold catalyst. The record turnover frequency (TOF) was as high as 2882 h-1 at a mild temperature of 50°C without any additives. The successful application of the heterogeneous gold catalyst for high-concentration formic acid dehydrogenation, will pave a way for rational design of new types of catalysts for energy storage and evolution by formic acid. The work has been recently published in Energy & Environmental Science (2015, 8, 3204-3207).
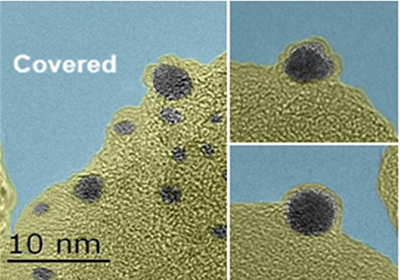
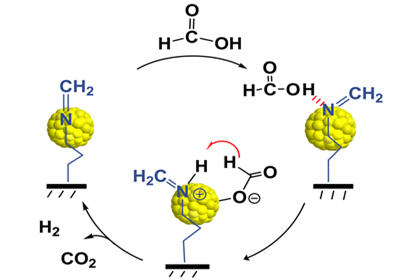
DICP Scientists Realize Pure Formic Acid Dehydrogenation by Using A Heterogeneous Schiff Base Functionalized Gold Catalyst(Photo by HUANG Yanqiang)
Hydrogen has been considered as one of the best candidates to satisfy the increasing demands for an efficient and clean energy supply. Controlled storage and release of hydrogen are the widely known technological barriers in the fuel cell based hydrogen economy. Recently, formic acid, was identified as a safe and convenient hydrogen carrier in fuel cells designed for portable use, due to its high energy density, non-toxicity and excellent stability. However, efficient heterogeneous catalysis for H2 production from formic acid dehydrogenation could only be achieved with the presence of additives, such as triethylamine and formate, or at a low concentration of formic acid (generally less than 1 M), which will not only decrease its hydrogen storage capacity, but also restrict its application in fuel cells due to the traces of amines volatilized. In the study, the unusual performance for pure formic acid dehydrogenation can be attributed to the synergistic mechanism between the Schiff base and gold nanoparticles at the interface, which facilitated the O-H bond dissociation and then undergo β-hydride elimination to produce CO2 and H2. Moreover, the formed gold nanoparticles with an encapsulating structure significantly increases the interfacial area between the Schiff base and gold NPs. Further studies about the unique mechanism between the Schiff base and gold as well as its application in the field of heterogeneous catalysis are ongoing in their lab.(Text/ Photo by HUANG Yanqiang)