The paper entitled “A local mode picture for H atom reaction with vibrationally excited H2O: A full dimensional state-to-state quantum dynamics investigation” was recently published online in Chemical Science
(http://pubs.rsc.org/en/content/articlelanding/2015/sc/c5sc03472h#!divAbstract),
written by Shu Liu and Pro. Dong. H Zhang.
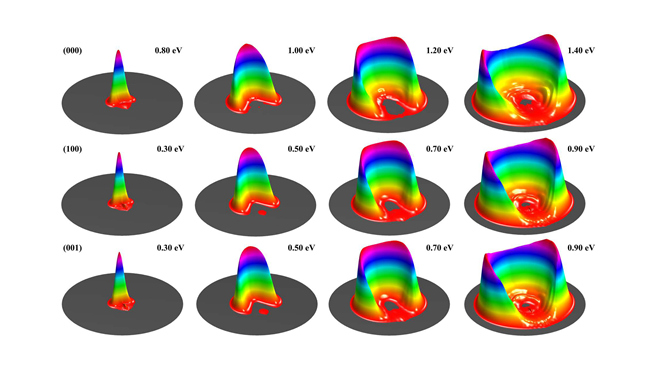
Normal mode picture has been extensively used to understand molecular vibrations. In the normal mode picture, the symmetric and asymmetric stretch excitations of H2O molecule look very differently, involving simultaneous excitation of both OH bonds. As a result, they were expected to behave differently when reacting with H atom, and produce OH with some vibrational excitation after the breaking of the other OH bond. However, Zare and coworkers observed negligible OD excitation from H reacting with D2O in fundamental asymmetrical excitation. A local mode picture was postulated to explain the phenomenon, but has never been confirmed.
DICP Researchers confirm the local mode picture for H atom reaction with vibrationally excited H2O(Photo by Shu LIU & Shuling CHEN)
DICP Researchers report the first full-dimensional state-to-state study for the title reaction with H2O in the ground and the first symmetric and asymmetric stretching excited states. It is found that symmetric and asymmetric stretching excited states behave essentially identically in the reaction. More importantly, their calculation revealed that the populations of OH in v=1 state from vibrational excited states are very close to the relative reactivity between the ground and vibrationally excited states, therefore verified the local mode picture for the reaction. In the local mode picture, the (100) and (001) states correspond to , i.e. each OH bond is a superposition of the ground and first excited quantum states. The incident H atom tends to react with excited state, producing OH mainly in corresponding ground state.(Text/Photo by Shu LIU & Shuling CHEN)