The ubiquity of C-H bonds renders arenes and alkanes ideal starting materials in organic syntheses. However, C-H bonds are generally inert; therefore metal-mediation is typically necessary. Recent studies have shown that Cp*Rh(III) complexes are powerful catalysts in activation of arene C-H bonds. Nevertheless, given the diversity of substrates and reaction patterns, activation of both C-H substrates and the coupling partners remains necessary. The 209 Group of DICP (supervised by Prof. Dr. Xingwei Li) has performed systematic studies in C-H activation, and has recently reviewed this exciting chemistry (Acc. Chem. Res. 2015, 48, 1007.). Below are recent representative projects.
(1) Activation of Arenes
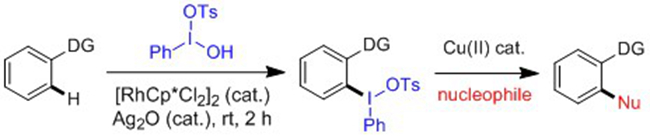
The carbon atoms in arene C-H bonds and in M-C species are nucleophilic and can only electronically match electrophiles. However, nucleophiles are more abundant in nature. To solve this limitation, the 209 Group managed to convert nucleophiles into electrophiles under in situ conditions, a process that is referred to as umpolung (J. Am. Chem. Soc. 2014, 136, 4780; Org. Lett. 2015, 17, 920.). In order to extend the concept of umpolung, they recently further realized rhodium(III)-catalyzed hyperiodination of arenes under mild conditions, leading to facile synthesis of reactive and unsymmetrical diaryliodoniums. This process represents rather unusual umpolung of the arene because the C--H of the arene was converted to C--I(III). These diaryliodoniums can be readily functionalized by a series of nucleophiles under copper catalysis, leading to facile construction of C-C, C-N, C-O, C-S, C-P and C-Br bonds under convergent conditions. This work has been published in Angew. Chem. Int. Ed. 2015, 54, 7405.
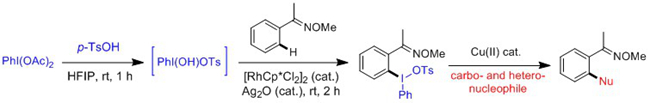
(2) Activation of sp3 C-H Bonds
The Rh(III)-catalyzed C-H activation are mostly restricted to C(sp2)-H substrates. In contrast, Rh-catalyzed activation of C(sp3)?H bonds is rather challenging because of the steric hindrance of C(sp3)?H bonds and the low reactivity of the resulting Rh-C(alkyl) species toward the coupling partner. On the other hand, nitrogenation via C-H activation represents an important strategy to access C-N bonds. The group of 209 recently reported rhodium(III)-catalyzed mild amidation of C(sp3)-H bonds in a broad scope of substrates including 8-methylquinoline, 8-benzylquinoline, and aliphatic cyclic and acyclic ketoximes (Angew. Chem. Int. Ed. 2015, 54, DOI: 10.1002/anie.201506323). In the context of C(sp3)-H activation reactions, the method is applicable to the late-stage functionalization of natural products and other complex molecules. A catalytic cycle has been proposed starting from C-H activation, decarboxylation of the amidating reagents to yield a nitrene and insertion into this transient nitrene. Experimental results also demonstrated that silver salts (AgOAc) likely both facilitated C-H activation and activated the amidating reagent.
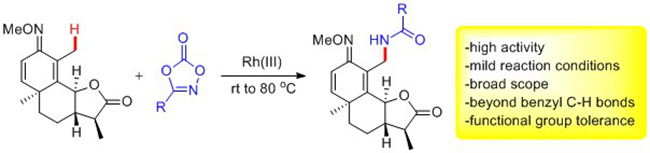
These works have been financial supported by the NSFC, the Chinese Academy of Sciences, and the Postdoctoral Science Foundation of China.