Recently, Prof. Zhou’s group made a significant progress in deracemization of secondary and tertiary amines with a tetrahydroisoquinoline core. This work is now online published in Journal of the American Chemical Society (J. Am. Chem. Soc.2015, 137, 10496-10499.).
New Research Progress in Deracemization of Secondary and Tertiary Amines(By Yue Ji)
Classical and kinetic resolution of racemic mixtures are the most widely used methods in large-scale preparation of enantiomerically pure compounds, but they are impeded by the fact that the theoretical yield of the target chiral molecule is only50% and at least half of the starting material would be discarded. Deracemization is a highly efficient technology to obtain enantiomerically purecompounds, especially when the substrate and the desired product possess an identical chemical structure.
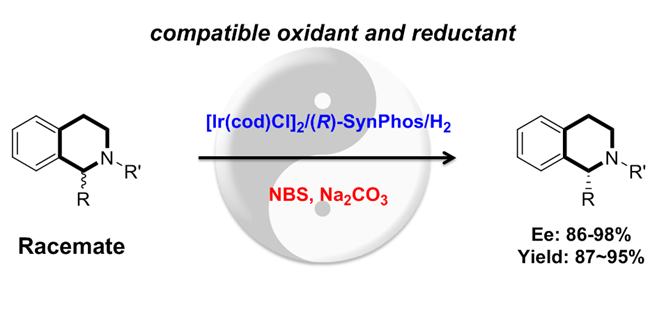
Deracemization reaction is comprised of two half-reactions that are opposite in reacting direction and completely distinct in mechanistic pathways, at least one should operate enantioselectively. Combination of oxidation with reduction is a common practice through destroying and regenerating of the chirality of stereogenic center. The main challenge of redox-driven deracemization is that oxidants and reductants easily and directly quench each other in single reactor, which usually is thermodynamically and kinetically favorable. Sequential operation or physical isolation of oxidation and reduction has been employed to overcome this problem.
Prof. Zhou’s group worked on asymmetric hydrogenation of heteroaromatic compounds with a series of important achievementsin the past decades, which inspired us in the deracemizations of amines. A concise deracemization of amines with tetrahydroisoquinoline core has been successfully realized by orchestrating a redox process consisted of N-bromosuccinimide(NBS) oxidation and iridum-catalyzed asymmetric hydrogenation.This rare compatible redox combination enables one-pot single-operation deracemization to generate chiral 1-substituted 1,2,3,4-tetrahydroisoquinolines with up to 98% ee and 95% yield, offering a simple and scalable synthetic technique for chiral amines directly from racemic starting materials.
This work was financially supported by the National Natural Science Foundation of China (21472188 and 21125208) and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2014167). (Text/Photo by Yue Ji)