Recently, Prof. Can Li`s group in State Key Laboratory of Catalysis and the solar energy division of Dalian National Laboratory of Clean Energy published new result on photoelectrical imaging of photogenerated charge separations on different facets of a single photocatalyst particle. Making use of the newly developed photoelectrical imaging technique, they gave the first direct evidence of highly anisotropic photogenerated charge separations on different facets of a single BiVO4 photocatalyst, under in situ photo illumination. The role of the built-in electric field in SCR of different facets played in the anisotropic photoinduced charge transfer in a single semiconductor crystal is clearly revealed. This work is now online published in the journal of Angewandte Chemie International Edition. (http://dx.doi.org/10.1002/anie.201504135)
Producing solar fuels by harvesting energy from sunlight offers promising approaches to solve the energy challenge with minimal environmental impact. Among these approaches, photocatalytic splitting of water into H2 and O2 in the presence of photocatalysts is recognized as an ideal way for utilizing the solar energy. Advancing these photocatalysts towards high light to energy conversion efficiency requires efficient separation of photogenerated electrons and holes. Focusing on this key issue, strategies and new concepts were proposed in Prof. Can Li’s group in the past few years: the fabrication of CdS/MoS2 heterojunction resulted in much higher photocatalytic activity of H2 evolution than CdS/Pt (J. Am. Chem. Soc., 2008, 130, 7176-7177);Surface-phase sensitive UV Raman spectroscopy revealed that, the photocatalytic activity of TiO2 can be greatly enhanced when anatase TiO2 nanoparticles were highly dispersed on the surface of rutile TiO2 to form anatase–rutile surface-phase junctions (Angew. Chem. Int. Ed., 120: 1766-1769 2008). Based on this concept, they further found the surface phase junction on Ga2O3 can significantly improve photocatalytic overall water splitting into H2 and O2. Ultra-fast transit absorption spectroscopy indicated that fast electron transfer (<3 ps) across the phase junction helped the charge separation (Angew. Chem. Int. Ed., 2012, 51, 13089-13092); Using monoclinic bismuth vanadate crystal as a model photocatalyst, they demonstrated that efficient charge separation can be achieved on different crystal facets, as evidenced by the reduction reaction with photogenerated electrons and oxidation reaction with photogenerated holes (Nature Comm., 2013,4,1432).
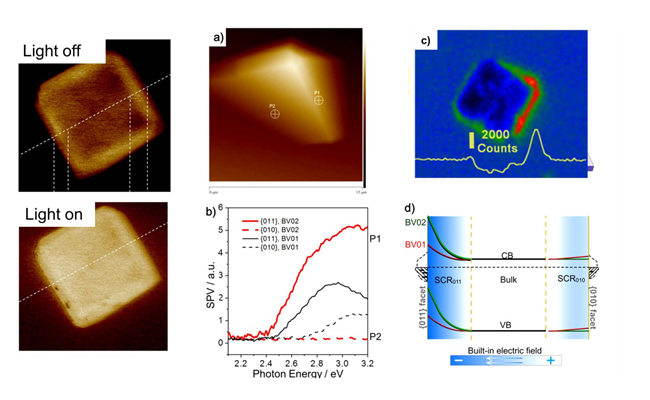
To further reveal the key issue of charge separation in these systems, they recently developed a new technique, Spatially Resolved Surface Photovoltage Spectroscopy (SRSPS) in combination with Kelvin Probe Force Microscopy (KPFM) to directly probe the spatial distribution of photogenerated charge from nanometer to micrometer scale, on the surface of photocatalysts. Making use of the newly developed technique, they gave the first direct evidence of highly anisotropic photogenerated charge separation on different facets of a single BiVO4 photocatalyst. Highly anisotropic photoinduced hole distribution was observed in single BiVO4 crystals with preferentially exposed {010} facets, and the SPV signal intensity on the {011} facet is 70 times stronger than that on the {010} facet. The role of built-in electric field in SCR of different facets played in the anisotropic photoinduced charge transfer in a single semiconductor crystal is clearly revealed. Moreover, single particle fluorescence microscopy provided further evidence to support this new finding. The result gives an insight into the nature of photogenerated charge separation in a single semiconductor photocatalyst particle at nano/micro meter scales. These findings provide the important scientific basis for developing highly efficient photocatalysts of hydrogen evolution, CO2 reduction and other artificial photosynthesis.
This work is supported by the major research plan of National Natural Science Foundation of China, Basic Research Program of China (973 project) and Collaborative Innovation Center of Chemistry for Energy Materials (2011·iChEM).
(By Fengtao Fan and Jian Zhu)