Nano and interfacial research group in the State Key Laboratory of Catalysis (SKLC) has recently made new research progress in catalysis under two-dimensional (2D) atomic crystal and in-situ dynamic surface characterization. These new results have been published in the recent issue of Nano Letters (Nano Lett 2015, 15, 3616-3623, doi: 10.1021/acs.nanolett.5b0120).
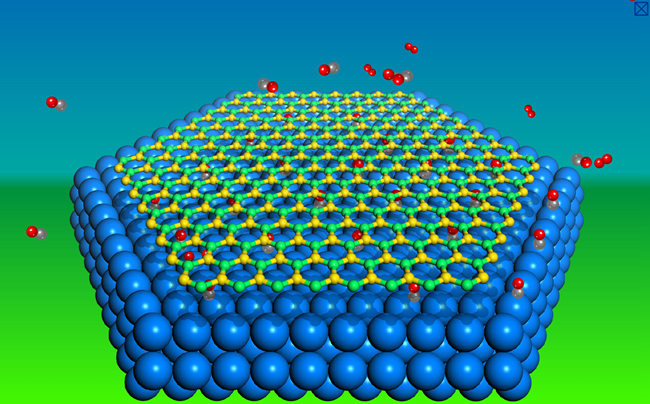
A team lead by Prof. Qiang Fu and Prof. Xinhe Bao makes use of the newly developed photoemission electron microscopy (PEEM)/low energy electron microscopy (LEEM) system in SKLC and other scientific instruments in Berkley National Laboratory, studying catalysis at graphene/metal interfaces. They find that the interface between the graphene overlayer and the metal surface can be regarded as a nanosized reactor and the graphene cover exerts a strong confinement effect on the surface catalysis. Accordingly, a concept “Catalysis under Cover” has been developed illustrating the 2D confinement effect on catalysis (Angew Chem Int Ed 2012, 51, 4856; PNAS 2014, 111,17023; Chin J Catal 2015, 36, 517). Recently, they have found that hexagonal boron nitride (h-BN) can exhibit more significant confinement effect than graphene. Cooperating with Prof. Mingshu Chen in Xiamen University, kinetics of CO oxidation reaction on the h-BN/Pt(111) surfacehas been investigated and enhanced reactivity of Pt surface has been observed due to the h-BN cover. These results suggest that catalytic performance of a metal catalyst can be substantially improved by covering the surface with a graphene or h-BN cover, which provides a new route towards modulation of metal catalyzed reactions.
This work is supported by the Natural Science Foundation of China, the Ministry of Science and Technology, the Chinese Academy of Sciences, the Ministry of Finance, and the iChEM program. (By Ying Shi)