Nano and interfacial research group in the State Key Laboratory of Catalysis (SKLC), in collaboration with Prof. Jianguo Wang from Zhejiang University of Technology,has recently made research progress in electrocatalytic reduction of CO2. They report that CO2 is efficiently reduced to CO over Pd nanoparticles with prominent size dependence. The results are published in Journal of the American Chemical Society (J. Am. Chem. Soc. 2015, 137, 4288-4291).
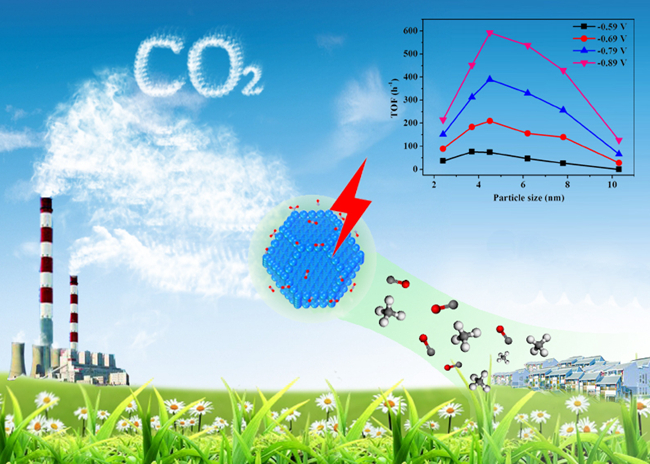
In recent years, increased carbon dioxide emissions posed a serious threat to the ecological environment for human survival.Traditional chemical methods for CO2 reductionneeds to provideenergy and hydrogen gas, whereaselectrocatalytic reduction of CO2, coupled with water electrolysis to obtain hydrogen from water, can be used to directly prepare carbon monoxide, hydrocarbon,methanol and other high value chemicals and liquid fuel under relatively mild reaction conditions in one step.At the same time, the process in combination with renewable energy and nuclear power can achieve large-scale energy storage, showing promising applications, which has become an important research focus in related fields.
Pd isa typical catalyst for hydrogen evolution reaction, and there are several fundamental challenges in theelectrocatalytic reduction of CO2 in bulk Pd electrode, such as high overpotential, low Faradic efficiency due to the competitive hydrogen evolution reaction (HER), etc. The experimental studies show that the Faradaic efficiency for CO production varies from 5.8% at -0.89 V (vs. reversible hydrogen electrode) over 10.3 nm NPs to 91.2% over 3.7 nm NPs, along with an 18.4 fold increase in current density. Based on the Gibbs free energy diagrams from density functional theory calculations, the adsorption of CO2 and the formation of key reaction intermediate COOH* are much easier on edge and corner sites than on terrace one of Pd NPs. In contrast, the formation of H* for competitive hydrogen evolution reaction is similar on all three sites. A volcano-like curve of the turnover frequency for CO production within the size range suggests that CO2 adsorption, COOH* formation and CO* removal during CO2 reduction can be tuned by varying the size of Pd nanoparticles, thus realizing the conversion from hydrogen production to efficient CO2 reduction overPd nanoparticles.
This work is supported by the Natural Science Foundation of China and the Ministry of Science and Technology. (By DunfengGao and Feng Jiao)