A critical review on catalytic synthesis of amines from alcohols and amines through borrowing hydrogen strategy was recently published in Chemical Society Reviews by Prof. Zhengkun Yu, et al. from the Laboratories of Organometallic Catalysis and Synthesis, Chinese Academy of Sciences.
http://pubs.rsc.org/en/content/articlepdf/2015/cs/c4cs00496e?page=search
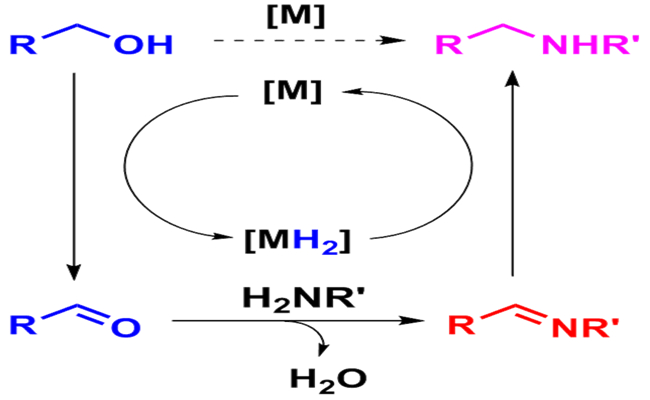
Transition metal-catalyzed N-alkylation of amines and related compounds with alcohols avoids the use of alkylating agents by means of borrowing hydrogen (BH) activation of the alcohol substrates. Water is produced as the only by-product, which makes the “BH” processes atom-economic and environmentally benign. Diverse types of homogeneous organometallic and heterogeneous transition metal catalysts, and substrates such as N-nucleophiles including amines, amides, sulfonamides and ammonia, and various alcohols, can be used for this purpose, demonstrating the promising potential of “BH” processes to replace the procedures using traditional alkylating agents in pharmaceutical and chemical industries. This critical review summarizes the recent advances in “BH” N-alkylation of amines with alcohols since 2009. “Semi-BH” N-alkylation processes with or without an external hydrogen acceptor is also briefly presented. Suitable discussion of the “BH” strategy provides new principles for establishing green processes to replace the relevant traditional synthetic methods for C-N bond formation. The recent achievements in this area from Dalian Institute of Chemical Physics was also highlighted. (Lab 203/Ping Wu)