Dr. Jianping Xiao, Profs. Xiulian Pan and Xinhe Bao from the State Key Laboratory of Catalysis at Dalian Institute of Chemical Physics recently made new progress on Confined Catalysis in Carbon Nanotubes. The work was chosen as a Spotlight article (J. Am. Chem. Soc. 2015, 137, 1?1) and just published by Journal of American Chemical Society (J. Am. Chem. Soc. 2015, 137, 477?482).
The series of experimental studies published by the group and also by other research groups over the years have demonstrated that the nanochannels created by curved graphene layers can modify the physiochemical properties of the encapsulated metal nanoparticles, alter the adsorptiona and activation of molecules and even their reaction pathways. This leads to modified catalytic activities, with some reactions enhanced whereas others hindered (PNAS 2013, 100, 14861; Angew. Chem. Int. Ed. 2013, 52, 317; Acc. Chem. Res. 2011, 44, 553; Energy Environ. Sci. 2011, 4, 4500; J. Am. Chem. Soc. 2008, 130, 9414; Nat. Mater. 2007, 6, 507).
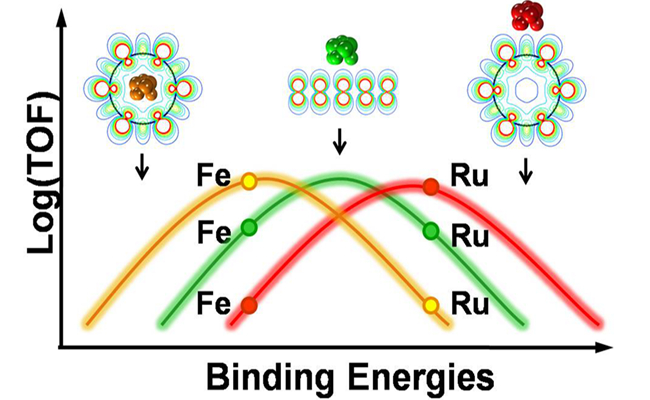
In order to understand the underlying mechanisms of the confinement effects on catalysis, the researchers at DICP now understaken a theoretical study combining density functional theory with the experiment results that have been rerported for several CNT-encapsulated transition metal catalysts. A new concept of confinement energy is proposed, which enables prediction of confinement effects on ctalytic activities in different reactions. They find that the nanospace and ? electrons – highly mobile electrons present in the curved graphene sheets that make up nanotubes-create a microenvironement, reducing the dissociative binding energy of several probe molecules such as CO, N2 and O2 with respect to those outside of the nanotube. For encapsulated iron, the weakened binding results in increased catalytic activity in CO hydrogenation, while for ruthenium the confinement results in the opposite effect in CO hydrogenation, NH3 synthesis and decomposition. These findings can be utilized to design efficient nanocatalysts for and modulate their activities for important processes.