Alkali metals have been widely employed as catalyst promoters, however, the promoting mechanism remains essentially unclear. Recently, researchers from Complex Hydride Materials Research Group (DNL1901) found that lithium, when in imide form, synergized with the iron nitride leading to superior catalytic activities in NH3 decomposition. Its activity is over one order of magnitude greater than those of Fe2N and Fe/CNTs at 723 K. Combined with XRD, XAFS, isotope labelling technique and theoretical simulations, a new alkali promotion mechanism was proposed. Li2NH, as an NH3 transmitting agent, favors the formation of higher N-content intermediate (LiTMN), where Li executes inductive effect to stabilize the TM-N bonding, and thus alters the reaction energetics. Such a discovery leads them to establish a novel catalyst system for NH3 decomposition, i.e., Li2NH-3d transition metals or their nitrides. Universal and unprecedentedly high catalytic activities are obtained over Li2NH-TM(N) spreading from Ti to Cu in NH3 decomposition. The overall catalytic activities of the Li2NH-TM(N) exhibit a volcano shape centered at Mn site, in contrast to the neat 3d TM(N) where Ni was found to have the best performance. More importantly, the Li2NH-MnN has an activity superior to that of the highly active Ru/CNTs catalyst. The present study may shed light on the design and development of more active and less expensive catalysts for chemical production and energy harvesting. The research paper has been highly recommended by the referees and published on line as VIP (very important paper) in Angew. Chem. Int. Ed., doi: 10.1002/anie.201410773, http://dx.doi.org/10.1002/anie.201410773.
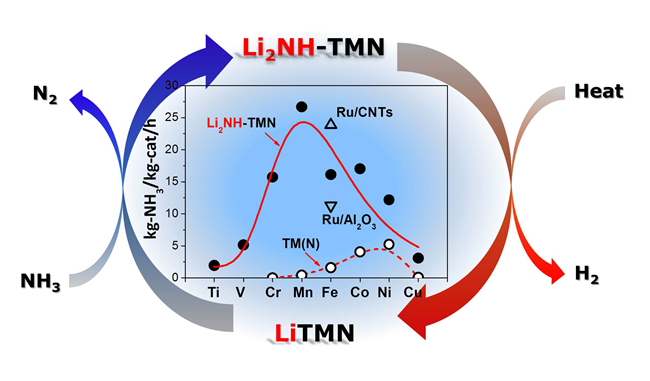
Alkali metal amides/imides have been successfully used in hydrogen storage. This work discloses another possibility of these materials in heterogeneous catalysis.
Catalyst characterization and theoretical understanding are derived from the collaborated research with Prof. Junhu WANG from DICP and Prof. Anan WU from Xiamen University, respectively. This work is supported by the National Science Fund for Distinguished Young Scholars and the National Natural Science Foundation of China (NSFC). (by Jianping GUO)