Search for low-cost and earth-abundant non-precious-metal catalysts to replace the rare precious metals and realize the high-efficient conversion of significant energy and chemical engineering processis a research focus in today’s catalysis science and chemical engineering fields. Recently, on the basis of previous studies towards nano-carbon catalysis,associate Prof. Dehui Deng and Prof. Xinhe Bao etc. from SKLC, DICP has innovated the preparation strategy of two-dimensional graphene-like materialsand successfullysynthesized ultrathin graphene shells (generally1-3 layers) encapsulating uniform 3dtransition metal nanoparticles. DFT calculations and experiments indicate that encapsulating the active metal nanoparticles into the graphene shells canefficientlyseparate the metal nanoparticles from the external harsh conditions(e.g. strong acid and base, and strong oxidant etc.), and therefore hinder the catalyst’s deactivation during the catalytic reaction process.Meanwhile, the active electron of encapsulated metal nanoparticles willpenetrate through the graphene layer to the outermost surface, thereby leading to a high catalytic activity on the graphene surface. The graphene shells encapsulating CoNi nanoalloys based on the theory showsa superior catalytic activity and durabilityfor electrocatalytic hydrogen evolution reaction (HER) in acidic medium with an overpotential of only 142 mV at the current density of 10 mA/cm2, which is quite close to the commercial 40% Pt/C.Thiswork has been recently published in Angewandte Chemie International Edition (Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201409524) and selected as a “Hot Paper” by the journal.
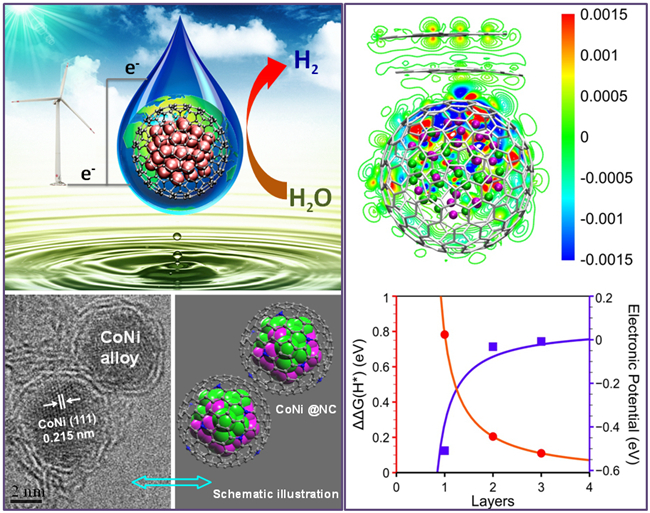
The concept of active metal nanoparticles protected by graphene shells and catalysis from penetrated electron has been developed for the first time when they investigated the iron nanoparticles encapsulated in podlike carbon nanotubes for the substitution of precious Pt in polymer electrolyte membranefuel cells (Angew. Chem. Int. Ed. 2013, 52, 371).It has been widely accepted by the international counterparts and described vividly as “chainmail for catalyst”. In recent years, rapid progress has been made towards this concept in electrocatalysis, photocatalysis and heterogeneous catalysis etc., and it has attracted great interests bymanyresearch groups.The Prof. Bao’s group who developed this concept initially has also madeseveral important progress, e.g.they found that the thickness of graphene shells (chainmail) can remarkably affect the electron penetration process and the catalytic oxygen reduction activity by experiments and DFT calculations (J. Mater. Chem. A 2013, 1, 14868).They extended the concept in electrocatalytic HER and proposed the HER mechanism on thegraphene surface (Energy Environ. Sci. 2014, 7, 1919). They collaborated with other research group to use a carbon encapsulated FeNi catalyst as the counter electrode material of dye-sensitized solar cells and found that the catalystshowed superior activity than the precious Pt in the reduction of I3- (Angew. Chem. Int. Ed. 2014, 53, 7023).
These works are supported by National Natural Science Foundation of China, Strategic Priority Research Program of the Chinese Academy of Sciences, and Collaborative Innovation Center of Chemistry for Energy Materials (2011 iChEM). (by Ying Shi and Jiao Deng)