The powers of energy for different degrees of freedom are different in driving a chemical reaction. The vibrational excitation effect of chemical reagent on chemical reactivity and dynamics is one of the most important research topics in the field of mode selective chemistry, bond selective chemistry and control of chemistry. Usually, the increasing energy is always helpful for promoting a chemical reaction. However, to the F + CHD3 reaction, it was found that the excitation of the C-H bond prohibits the breaking of the C-H bond. In order to provide further insights, a quantitative experimental study is required as a challenge.
A detailed experimental study on this reaction has been carried out by Yang’s group using crossed-beam time-slicing velocity map ion imaging method (Rev. Sci. Instrum. 79, 094104 (2008)). Quantitative information of the vibrational excitation effects on the chemical reactivity and dynamics of this reaction was obtained. It was found that this reaction is suppressed by the C-H stretching excitation with an overall suppression of 26%. The vibrational energy in the exciting C-H bond is exclusively deposited into the HF product vibration, while the vibrational state distribution of the CD3 products is only slightly affected. Currently, this work provides the best experimental data set comparing with further theoretical studies.
Recently, the paper “How is C-H Vibrational Energy Redistributed in
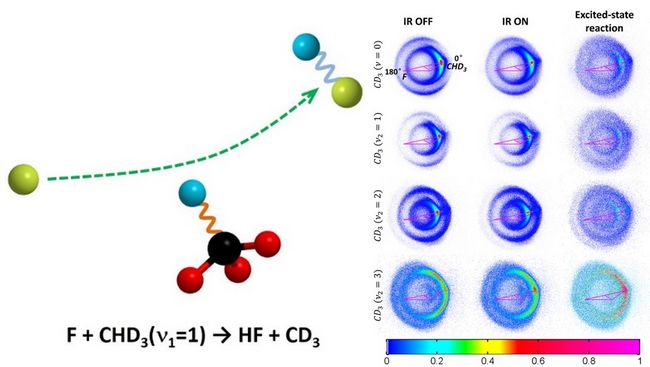
Fig. Reaction mechanism scheme (left panel) and acquired images of CD3 products (right panel).
Contact: Guorong Wu
Email: wugr@dicp.ac.cn