Recently, the research team headed by Prof. Hanfa Zou in CAS Key Lab of Separation Sciences for Analytical Chemistry, National Chromatographic R&A Center, Dalian Institute of Chemical Physics, Chinese Academy of Sciences applied quantitative proteomics to investigate the kinetics of trypsin digestion. They found that protein digestion priority is independent of protein abundances. This work was published as correspondence in a new issue of Nature Methods (Nature Methods, 2014, 11,220-222).
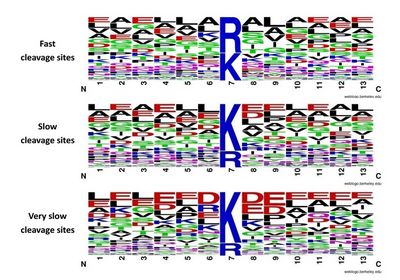
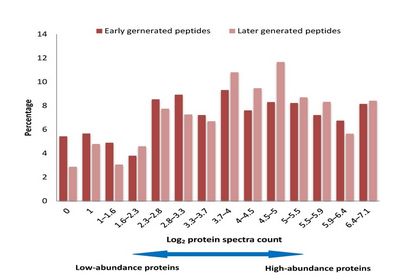
Protease is often used to digest proteins into peptide for high throughput proteomics analysis. There are huge number of proteins with different abundances coexisted in the proteome sample. When a protease is added into the proteome sample for digestion, it will hydrolyze the peptide bonds on these proteins. One intersting issue is which type of proteins, the high-abundance proteins or low-abundance proteins, would be digested firstly. To answer this question, Mingliang Ye, Hanfa Zou et al have designed a quantitative proteomics appraoch to investigate the kinetics of trypsin digestion. They compared the abundance of generated peptides for a two step digestion expriments. Their results indicated that (1) the kinetics of cleavage site depends on the amino acid residue surrounded, the sites surrounded with neutral residues will be cleaved quickly while the sites surrounded with charged residues will be cleaved slowly; (2) the digestion prority has no notable correlation with protein aboundance indicating that the high-abundance proteins are not digested firstly. The study indicated that the digestion priorities of individual proteins in a proteome sample depend mainly on their sequences but not on their abundances.
In proteomics study, the protein digestion, enzyme substrate screening all involve enzymatic reactions with huge number of protein/peptide substrates with diffent abundance. However, the enzamatic kinetics in such complex systems was not fully understood. This study indicated that quantitative proteomics could be an important tool for study of complex enzymatic kinetics because the dynamics of the generated products could be monitored in a high throughput way. And the study in this aspect will be an important complement to the classic Michaelis-Menten kinetics.(by Ye Mingliang)