A review article, entitled Morphology-dependent nanocatalysts: Rod-shaped oxides, has been recently published in Chemical Society Reviews (Chem. Soc. Rev. 43 (2014) 1543-1574) by the group of Prof. Wenjie Shen from the State Key Laboratory of Catalysis.
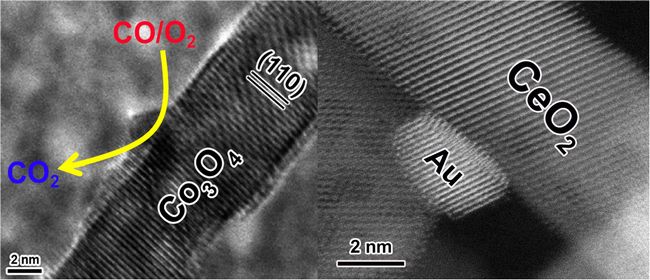
Nanocatalysts are characterized by the unique nanoscale properties that originate from their highly reduced dimensions. Extensive studies over the past few decades have demonstrated that the size and shape of a catalyst particle at the nanometer scale profoundly affect its reaction performance. In particular, controlling the catalyst particle morphology allows a selective exposure of a larger fraction of the reactive facets on which the active sites can be enriched and tuned. This desirable surface coordination of catalytically active atoms or domains substantially improves catalytic activity, selectivity, and stability. This phenomenon is called morphology-dependent nanocatalysts: catalyst particles with anisotropic morphologies on the nanometer scale greatly affect the reaction performance by selectively exposing the desired facets.
This review highlights important progress in morphology-dependent nanocatalysts based on the use of rod-shaped metal oxides with characteristic redox and acid–base features. The correlation between the catalytic properties and the exposed facets verifies the chemical nature of the morphology effect. Moreover, an overview of the interactions between the rod-shaped oxides and the metal nanoparticles in metal-oxide catalyst systems, involving crystal-facet-selective deposition of metal particles onto different crystal facets in the oxide supports, we provided. A fundamental understanding of active sites in morphologically tuneable oxides enclosed by the desired reactive facets is expected to direct the development of highly efficient nanocatalysts.(by Ning Wang)