The DICP Research Group led by Prof. YANG Xueming has carried out a systematic study on “Molecular Hydrogen Formation from Photocatalysis of Methanol on Anatase-TiO2(101).” The results were published in the recent issue of the recent issue of the Journal of American Chemical Society(J. Am. Chem. Soc., 2014, 136 (2), 602–605) .
Rutile and anatase TiO2 materials have been often used in the study of hydrogen (H2) production from photocatalytic water splitting, and previous study found that adding methanol (CH3OH) to pure water solution can dramatically enhance H2 production, implying that CH3OH played a crucial role in H2 production. Despite lots of work focusing on rutile (R)-TiO2, the anatase form is the most active polymorph in commercial applications for catalysis actually. Even though a lot of photocatslysis studies have been carried out on anatase (A)-TiO2 particles, very little was done on well-defined surfaces.
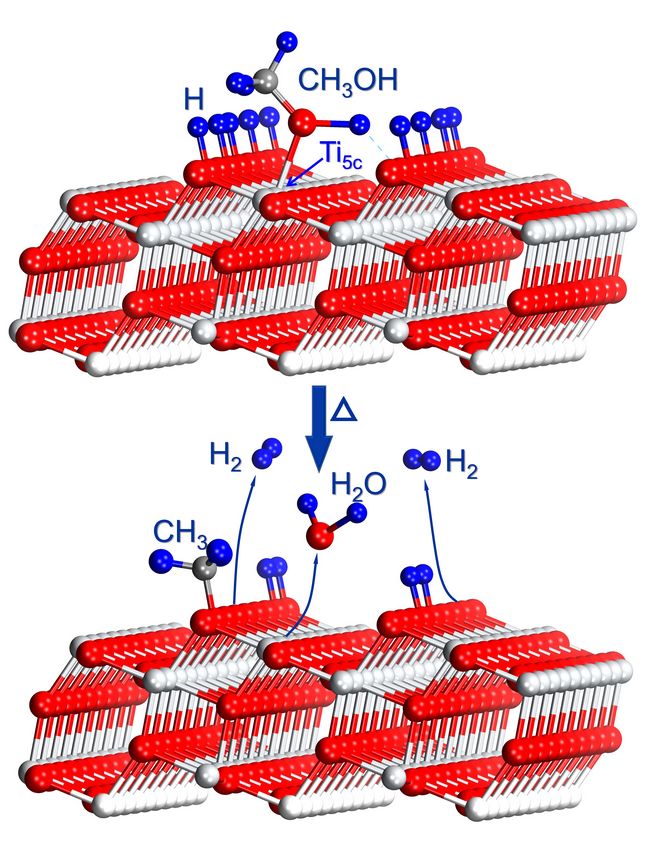
In a recent study, we have shown that photocatalytic dissociation of CH3OH on TiO2(110) occurs in a stepwise manner.( J. Am. Chem. Soc., 2012, 134 (32), 13366–13373). And D2 can be formed via thermal recombination of dissociated D-atoms on the bridge bonded oxygen (BBO) sites from photocatalysis of fully deuterated CH3OH, suggesting that the last molecular D2 formation step on the R-TiO2(110) surface is a thermally driven process. Since only about 7% of D-atoms on the BBO sites contribute to D2 formation with most D-atoms recombining with BBO atoms to form D2O, it implies that H2 formation is not the most efficient process on R-TiO2(110). (J. Am. Chem. Soc., 2013, 135 (28), 10206–10209). In this work, we have investigated molecular H2 formation on A-TiO2(101) from photocatalysis of CH3OH. Our results show that H2 is formed via thermal recombination of dissociated H-atoms on the BBO sites from photocatalysis of CH3OH on A-TiO2(101). The H atoms at the OBBO sites mostly come off as H2 on the A-TiO2(101) surface, not taking an OBBO to form H2O (the OBBO atoms on A-TiO2(101) are much more stable than on R-TiO2(110)). About 40% of the H atoms at the OBBO sites come off as H2 on A-TiO2(101), While only 7% of D-atoms on the BBO sites on R-TiO2(110) contribute to D2 formation, suggesting that the H2 formation process is more efficient on A-TiO2(101). The present investigation of the mechanism of H2 formation from UV CH3OH photocatalysis on A-TiO2(101) could help us to understand the nature of H2 production on TiO2 photocatalysts. (By XU Chenbiao and GUO Qing)