Aqueous zinc-based batteries (ZBBs) are widely used for portable and grid-scale applications due to their high safety, low cost and high energy density.
However, the inhomogeneous zinc deposition on anode during charging and the zinc dendrite formation decrease the cycling stability of ZBBs. Moreover, the traditional aqueous electrolytes are not capable of working at low temperature due to the suddenly dropped ionic conductivities, limiting the applicable temperature range of aqueous ZBBs.
Recently a research group led by Prof. LI Xianfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a low-temperature resisting, cost-effective, safe and eco-friendly hybrid electrolyte for aqueous ZBBs.
This work was published in Energy & Environmental Science.
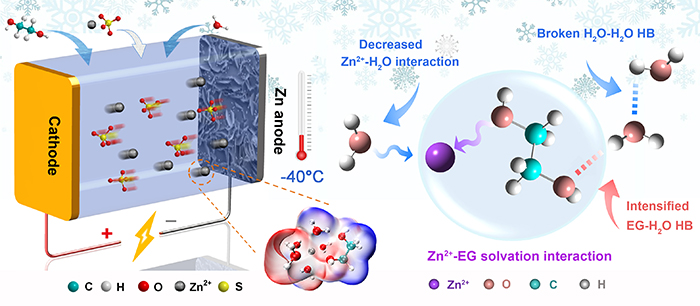
Schematic illustration of the ZBBs with hybrid electrolytes at low temperatures and possible mechanism of how Zn2+-EG solvation interaction impacts the chemistry of the hybrid electrolyte (Image by CHANG Nana)
The developed electrolyte, consisting of water (H2O), ethylene glycol (EG) and zinc sulfate (ZnSO4), exhibited high zinc-ion conductivity at low temperature.
"We demonstrated the unique solvation interaction of Zn2+ with EG through experiments together with theoretical calculation," said Prof. LI.
This interaction could not only enhance the hydrogen bonding between EG and H2O, providing the hybrid electrolyte with lower freezing point, but also weaken the solvation interaction of Zn2+ with H2O, achieving highly reversible Zn/Zn2+ chemistry and uniform zinc deposition.
Both the Zn-ion hybrid supercapacitors (ZHSCs) and Zn-ion batteries (ZIBs) with the hybrid electrolytes showed high energy densities, high power densities and long-cycle life at -20 °C. This series of hybrid electrolytes with tunable EG-to-H2O ratios provided good balance between performance and cost, which enabled promising application in various regions.
This work offers enlightenment for designing electrolytes for low-temperature energy storage devices. It was supported by the Natural Science Foundation of China and CAS Engineering Laboratory for Electrochemical Energy Storage. (Text by CHANG Nana)