More than 350 million tonnes of lower olefins are produced per year through the steam cracking of hydrocarbons. It consumes lots of energy to separate large quantities of chemical mixtures into pure accounts.
The efficient removal of alkyne impurities to produce polymer-grade lower olefins remains challenging.
A research team led by Prof. JIANG Ling from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, in collaboration with Prof. LI Landong from Nankai University and Prof. YANG Sihai from the University of Manchester, reported a strategy to control the pore interior of faujasite (FAU) zeolites and thus improve chemoselective alkyne/olefin separations. The results were published in Science.
They revealed that the reversible, chemoselective binding of alkynes on open Ni(II) sites confined in Faujasite (FAU) zeolites enables the efficient production of polymer-grade lower olefins under ambient conditions.
Under ambient conditions, Ni@FAU showed exceptional adsorption of alkynes and efficient separations of acetylene/ethylene, propyne/propylene and butyne/1,3-butadiene mixtures with unprecedented dynamic separation selectivities of 100, 92, and 83, respectively.
Prof. JIANG’s group developed an infrared photodissociation spectroscopy for in-situ and sensitive detection of key reaction intermediates. The adsorption species in C2H2- and C2H4- loaded Ni@FAU were identified by infrared photodissociation spectroscopy.
Their results indicated that by confining atomically dispersed Ni(II) sites into the FAU zeolite channels, the discrimination between alkyne and olefin binding was amplified in Ni@FAU, which enabled the production of polymer-grade olefins under conditions relevant to practical processes.
Combining its facile synthesis at large scale and excellent stability, the Ni@FAU sorbent offers a potential practical solution to the challenging alkyne/olefin separations.
The above work was supported by the National Natural Science Foundation of China. (Text and Image by WANG Chong)
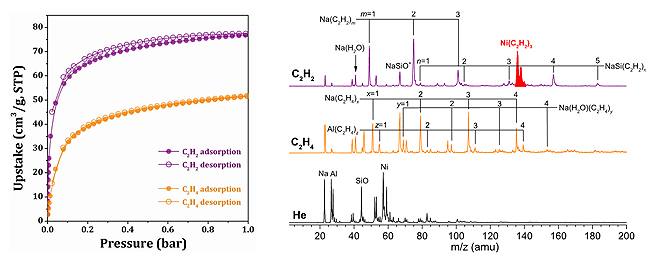
Adsorption isotherms of C2H2 and C2H4 for Ni@FAU (Left). Mass spectra of species produced by pulsed laser vaporization of the Ni@FAU target (Right). (Image by WANG Chong)