A research group led by Prof. WANG Feng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences recently developed a photocatalytic method for the conversion of biopolyols and sugars to methanol and syngas. The results were published in Nature Communications.
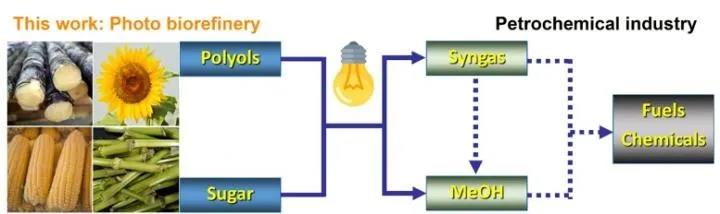
Methanol and syngas act as the platform chemical conecting the boirefinery and petrochemical industry.
Methanol is considered to be the most promising clean liquid fuel for the future and one that can be deployed on a large scale. In addition, it's a fundamental chemical material used for industrial production of ethylene and propylene. Currently, methanol is industrially produced from natural gas and coal.
Production of methanol from renewable and abundant carbon resources rather than fossils is a promising route. The bio-derived syngas to fabricate biomethanol is traditionally produced via gasification at high temperature (700-1000 °C). The process usually generates a mixture of CO, CO2, hydrocarbons and deficient H2 as well as coke, char and tar.
In the current study, the researchers converted biomass-derived polyols and sugars into methanol and syngas (CO+H2) via UV light irradiation at room temperature. The bio-syngas could be further used for the synthesis of methanol.
Cellulose and even raw wood sawdust can be converted into methanol or syngas after hydrogenolysis or hydrolysis pretreatment.
The researchers also found that Cu dispersed on titanium oxide nanorods (TNR) rich in defects effectively promoted selective C-C bond cleavage that produced methanol. Using this process, methanol was obtained from glycerol with co-production of H2. A syngas with CO selectivity up to 90% in the gas phase was obtained by controlling the energy band structure of Cu/TNR. The gas product could be facially tuned from CO2 to CO by controlling the energy band structure of Cu/TNR.