Recently, a research team led by Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences and their collaborators have achieved highly efficient H2 production from electrocatalytic decomposition of H2S via a robust graphene encapsulating metal catalyst. It provides a promising way for eliminating pollutant hydrogen sulfide and producing green hydrogen energy. These findings were published as a communication in the Energy & Environmental Science.
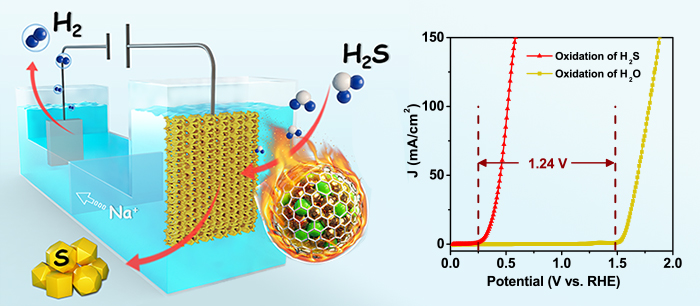
(Left): Electrocatalytic decomposition of H2S for H2 production via robust chainmail catalyst.
(Right): Comparison of sulfide oxidation reaction (SOR) and oxygen evolution reaction (OER) polarization curves for catalyst. The difference value of two reactions’ onset potential is 1.24 V.(Image by ZHANG Mo and GAO Hehua)
(Right): Comparison of sulfide oxidation reaction (SOR) and oxygen evolution reaction (OER) polarization curves for catalyst. The difference value of two reactions’ onset potential is 1.24 V.(Image by ZHANG Mo and GAO Hehua)
Hydrogen sulfide is a toxic by-product of oil and gas production released in enormous quantities, and it is also a potential source of an important green energy carrier, namely, hydrogen gas. Based on the Claus process, the current industrial technology for removing H2S only recovered sulfur from H2S, while the hydrogen constituent is wasted as steam. The electrocatalytic decomposition of H2S is a kind of mild and efficient method, which produces hydrogen at the cathode and recycle sulfur at the anode simultaneously.
However, this technique is hindered by lacking suitable catalysts, as the reported precious materials are high-cost and the bare non-precious metal materials are easily corroded. Therefore, developing a low-cost, high-efficient and robust electrocatalyst is crucial to achieve a sustainable electrocatalytic decomposition of H2S.
Based on the concept of chainmail catalysts which is firstly proposed by DENG’s group, (Angew. Chem. Int. Ed., 2013, 52, 371, Angew. Chem. Int. Ed., 2014, 53, 7023,Energy Environ. Sci., 2014, 7, 1919, Angew. Chem. Int. Ed., 2015, 54, 2100, Nature Nanotech., 2016, 11, 218, Energy Environ. Sci., 2016, 9, 123, Adv. Mater., 2017, 29, 1606967, Adv. Mater., 2019, 31, 1901996), researches from DENG’s group developed a kind of new graphene-encapsulated non-precious metal chainmail material as a low-cost and high activity electrocatalyst for decomposition of H2S.
The optimized catalyst drove hydrogen production from decomposing H2S at a much lower potential, which decreases 1.24 V than that of water splitting. Meanwhile, it displayed high activity which is almost twice current density than that of Pt/C, and maintained 500 h stability without decay.
Further the reaction active sites were studied by DFT calculations cooperating with Prof. GUAN Jing from Qingdao University of Technology. They demonstrated the superiority of catalyst originates from the modulation of the electronic structure of graphene surface by metal core and nitrogen dopant by the combination of theory and experiment results.
To demonstrate the reliability of this catalyst, the researches applied it into the demo of converting H2S from industrial syngas (2% H2S), which couplings the removal of H2S impurities and the production of hydrogen. The chainmail catalyst showed high stability during 1200 h test, indicating its potential for converting waste to sustainable energy in the practical application. In addition, there are a large amount of sulfide existing in the wastewater of paper mill and tannery. The development of this chainmail provides a new idea for electrocatalytic treatment for such kind of industrial wastewater.
These works were supported by Ministry of Science and Technology of China, National Natural Science Foundation of China, Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, the DNL Cooperation Fund and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM). (Text and image by ZHANG Mo and GAO Hehua)