Nano and interfacial research group in the State Key Laboratory of Catalysis (SKLC) has recently made new research progress in graphene confined catalysis and in-situ surface catalysis. The results are published at “Proceedings of the National Academy of Sciences of the United States of America” (PNAS) (PNAS 2014, 111 (48), p17023-17028, http://www.pnas.org/content/111/48/17023).
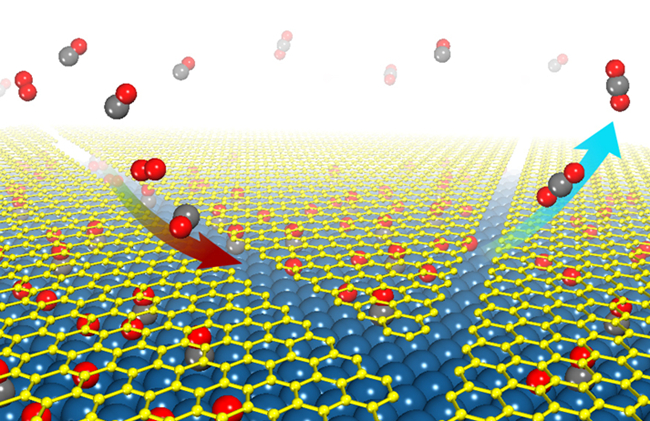
Making use of the newly developed photoemission electron microscopy (PEEM)/low energy electron microscopy (LEEM) system in SKLC and other scientific instruments in Berkley National Laboratory and Texas A&M University, Yunxi Yao, Qiang Fu, Xinhe Bao, and other coworkers have performed comprehensive studies in the chemistry under the graphene cover. Based on the understanding of the weak interaction between graphene overlayers and metal surfaces (Angew Chem Int Ed 2012, 51, 4856), they have further suggested that the two-dimensional (2D) space between graphene and metal can be regarded as nanosized reactor. Surface science measurements combined with density functional theory calculations show that CO and O2 molecules can feasibly penetrate into the graphene/metal interfaces under near ambient pressure conditions. Moreover, the grapheneoverlayer weakens the strong interaction between CO and Pt and,consequently, facilitates the CO oxidation with lower apparentactivation energy due to the confinement effect exerted by the graphene cover. In heterogeneous catalysis molecule-metal interaction has often been modulated through structural modification at the surface or under the surface of the metal catalyst. The present work suggests an alternative way towardsthis modulation by placinga 2D cover on the metal surface.
This work is supported by the Natural Science Foundation of China, the Ministry of Science and Technology, the Chinese Academy of Sciences, and the Ministry of Finance. (By Ying Shi)